Mikrolaks rectal solution tube 5 ml with universal tip No. 12

Instructions for Mikrolaks rectal solution, tube 5 ml with universal tip No. 12
Composition
active ingredients: sodium citrate, sodium lauryl sulfoacetate 70%, sorbitol solution 70%, crystallizing;
1 ml of rectal solution contains: sodium citrate 90 mg, sodium lauryl sulfoacetate 70% 12.9 mg, sorbitol solution 70%, crystallizing, 893 mg;
excipients: sorbic acid, glycerin, purified water.
Dosage form
Rectal solution.
Main physicochemical properties: viscous opalescent colorless solution containing small air bubbles.
Pharmacotherapeutic group
Drugs affecting the digestive system and metabolism. Laxatives in enemas. ATX code A06A G11.
Pharmacological properties
Pharmacodynamics.
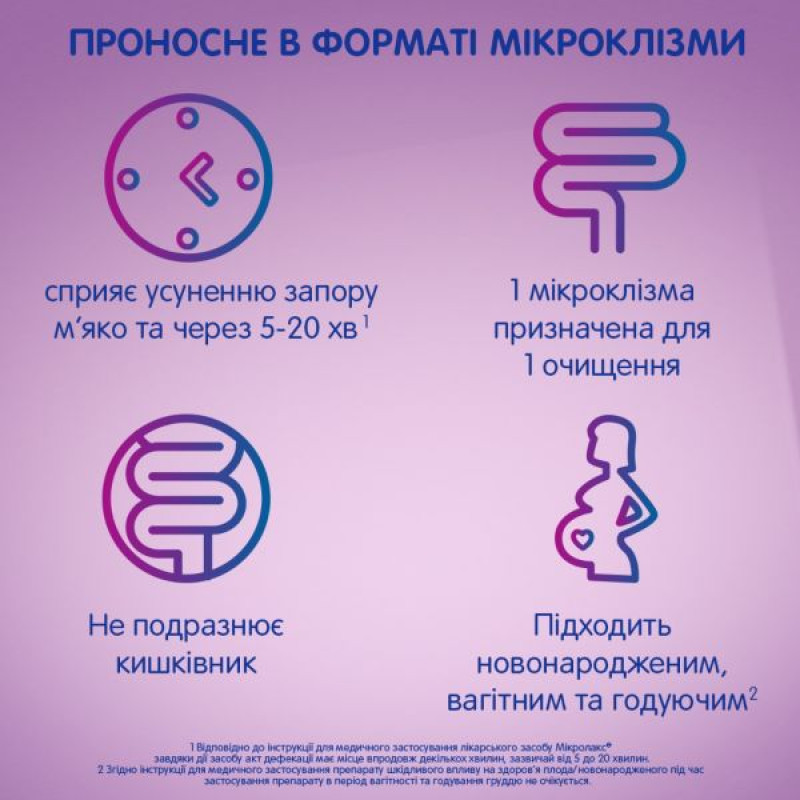
Mikrolaks® has a chemical and mechanical effect. Sodium citrate displaces bound water contained in the stool. Due to the sorbitol of the crystallizing solution, the effect of sodium citrate on the displacement of water is enhanced. Sodium lauryl sulfoacetate is a non-toxic, non-irritating wetting agent. It facilitates the penetration of the rectal solution into the stool. This process helps to liquefy the stool and allows the patient suffering from constipation to perform the act of defecation within a few minutes, usually from 5 to 20 minutes. The process of displacing bound water by chemical and mechanical means is called peptization. The increase in the amount of water due to peptization and liquefaction helps to soften the stool and facilitate bowel movements.
Indication
Constipation (short-term use), as well as diseases requiring easier defecation.
For bowel emptying during diagnostic and therapeutic procedures in the rectal area.
Contraindication
Hypersensitivity to the active substances or any excipients.
Do not use in case of intestinal obstruction or abdominal pain of unknown etiology.
Diagnosing hereditary fructose intolerance (intolerance to a certain type of sugar).
Concomitant treatment with calcium or sodium polystyrene sulfonate cation exchange resin (see section "Interaction with other medicinal products and other types of interactions").
Interaction with other medicinal products and other types of interactions
Studies on the interaction of Mikrolaks® with other drugs have not been conducted.
Since Mikrolaks® contains sorbitol, it is not recommended to use it simultaneously with ion exchange resins used orally or rectally for the treatment of hyperkalemia, due to the risk of intestinal necrosis.
Application features
Sorbic acid may cause irritation of the mucous membrane.
If symptoms persist for a long time, you should consult a doctor and avoid long-term use of the drug.
If the contents of the tube are only partially used, the remaining rectal solution must be disposed of.
Use during pregnancy or breastfeeding
Studies in pregnant women have not been conducted. Since the drug, when used as recommended, is likely to have only minor systemic absorption, no harmful effects on the health of the fetus/newborn are expected when the drug is used during pregnancy and breastfeeding.
It is not known whether sodium citrate, sodium lauryl sulfoacetate and sorbitol pass into human breast milk. During breastfeeding, the use of the drug is recommended after consulting a doctor and assessing the risk/benefit to the child.
Ability to influence reaction speed when driving vehicles or other mechanisms
Unknown.
Method of administration and doses
The drug is used rectally.
A single dose of the drug should be administered 5–20 minutes before the desired effect.
Adults and children over 3 years of age should use the contents of 1 tube of microenema with a universal tip (5 ml), inserting the microenema tip to its full length.
Newborns and children under 3 years of age should use the contents of 1 microenema tube with a universal tip (5 ml) and insert the microenema tip half way (see the marking on the tube tip).
Newborns and children under 3 years of age should use the contents of 1 microenema tube with a shortened tip (5 ml) and insert the microenema tip to its full length.
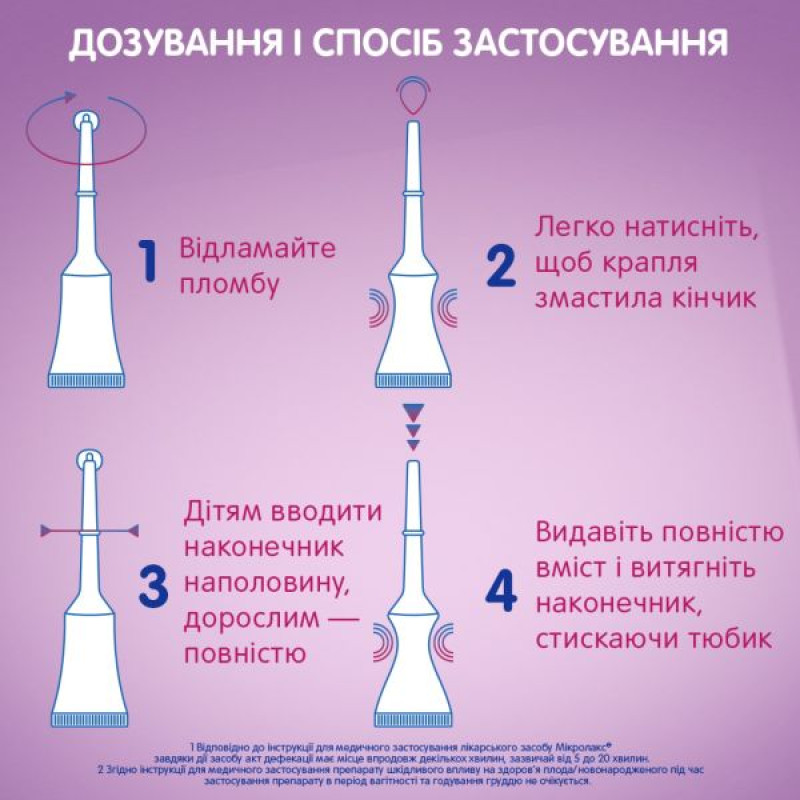
Application instruction
Break off the seal on the tip of the tube.
Press the tube lightly so that a drop of the drug lubricates the tip of the microenema tip to improve the administration process. Insert the universal microenema tip to its full length (for children under 3 years old - half the length) into the rectum. Squeeze the tube to completely squeeze out its contents. Remove the tip while continuing to squeeze the tube.
For children under 3 years of age, it is also possible to use a microenema with a shortened tip. In this case, it is necessary to enter the entire contents of one microenema tube rectally, inserting the tip to its full length.
Children
Used for children from birth.
Overdose
No cases of overdose have been reported to date.
Side effects
Adverse reactions identified during post-marketing use of sorbitol, sodium citrate and sodium lauryl sulfoacetate are listed in the table below. The frequency of adverse reactions is defined according to the following categories:
Very common (≥ 1/10)
Common (≥ 1/100 and < 1/10)
Uncommon (≥ 1/1000 and < 1/100)
Rare (≥ 1/10,000 and < 1/1,000)
Very rare (< 1/10,000)
Unknown (cannot be estimated from available data).
Organ system Frequency category | Side effect |
Immune system disorders Unknown | Hypersensitivity reactions (e.g. urticaria) |
Digestive system disorders Unknown | Abdominal pain Discomfort in the anorectal area Loose stools |
a Includes the preferred terms “Abdominal discomfort”, “Abdominal pain” and “Upper abdominal pain”.
Expiration date
3 years.
Storage conditions
Store at a temperature not exceeding 25 °C in a place inaccessible to children.
Packaging
Solution for rectal administration, 5 ml.
5 ml of rectal solution in a tube with a tip for single administration (polyethylene tube with a shortened or universal tip with a seal on the tip of the tube).
4 or 12 tubes with shortened tips in a cardboard box with labeling in Ukrainian.
4 or 12 tubes with universal tips in a cardboard box with labeling in Ukrainian.
Vacation category
Without a prescription.
Producer
DELPHARM ORLEANS, France.
Location of the manufacturer and its business address.
5 avenue de Concyr, ORLEANS CEDEX 2, 45071, France/5 avenue de Concyr, ORLEANS CEDEX 2, 45071, France.
Applicant
McNeil Products Limited.
Applicant's location
50-100 Holmes Farm Way, High Wakeham, HP12 4EG, England/
50-100 Holmers Farm Way, High Wycombe, HP12 4EG, England
Applicant's representative
Johnson & Johnson Ukraine LLC
Location of the applicant's representative
01010, Kyiv, 32/2 Ostrozkyh Knyaziv St., Ukraine.
+38 (044) 498 0888
+38 (044) 498 7392
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.