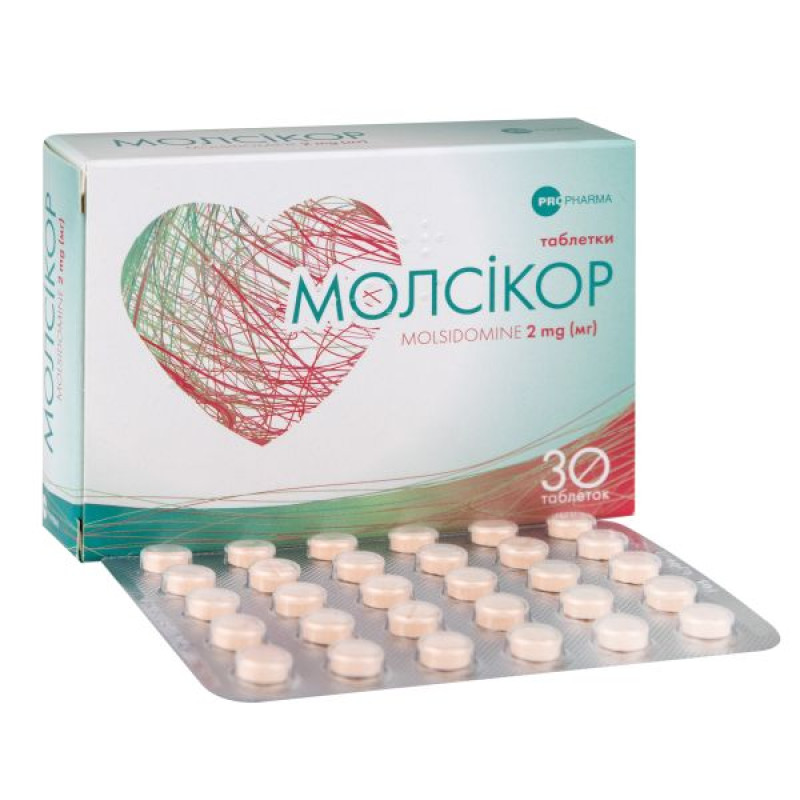
Molsicor tablets 2 mg blister No. 30

Instructions for use Molsicor tablets 2 mg blister No. 30
Composition
active ingredient: molsidomine;
1 tablet contains molsidomine 2 mg;
excipients: lactose monohydrate; sucrose; potato starch; orange-yellow S (E 110); povidone K-25; magnesium stearate.
Dosage form
Pills.
Main physicochemical properties: tablets of heterogeneous light orange color, round, flat on both sides with a bevel, with a dividing line on one side.
Pharmacotherapeutic group
Vasodilators used in the treatment of heart disease. Molsidomine. ATX code C01D X12.
Pharmacological properties
Pharmacodynamics.
Molsidomine is a derivative of sydnonimine. The active metabolite of molsidomine, linsidomine (SIN 1A), is a compound that reduces the tone of the smooth muscles of the vascular walls and has an antianginal effect. Relaxation of smooth muscles contributes to an increase in the volume of veins and the volume of the vascular bed, which leads to a decrease in venous return, due to which the filling pressure of both ventricles decreases. This reduces the load on the heart and improves hemodynamic conditions in the coronary circulation. The expansion of arterial vessels leads to a decrease in peripheral resistance, which reduces the load on the heart, reduces myocardial tension and, as a result, reduces the need for myocardial oxygen. In addition, molsidomine reduces spasm of the coronary arteries and dilates their large branches. The antiplatelet effect of molsidomine is of clinical importance in the treatment of ischemic heart disease. Unlike nitrates, molsidomine does not cause tachyphylaxis.
Pharmacokinetics.
After oral administration, molsidomine is absorbed in the gastrointestinal tract by approximately 90%. The onset of action is observed approximately 20 minutes after administration, the duration of action after a single dose is 4-6 hours. The maximum concentration in the blood is observed approximately 30-60 minutes. Bioavailability is approximately 65%, and it is 11% bound to plasma proteins. Molsidomine is biotransformed in the liver enzymatically to the active metabolite - sydnonimine 1 (SIN-1), which is non-enzymatically transformed to N-morpholino-N-aminoacetonitrile (SIN 1A) - linsidomine.
It is not known whether molsidomine and its metabolites pass into breast milk.
Molsidomine is primarily excreted in the urine – 90-95% (2% – unchanged) and feces – 3-4%. Total clearance is 40-80 l/h, and SIN-1 – 170 l/h. The half-life of the drug is 1.6 hours, and in case of severe liver failure it increases (in case of cirrhosis of the liver – approximately 13.1 hours). The half-life of the metabolite linsidomine is 1-2 hours and increases in case of severe liver failure up to 7.5 hours. The drug does not accumulate in the body.
Indication
Prevention and long-term treatment of angina pectoris when other drugs are contraindicated, not tolerated or have proven insufficiently effective, as well as for elderly patients.
Due to the delayed onset of action, the drug Molsicor is not suitable for the relief of an acute attack of angina pectoris.
Contraindication
Hypersensitivity to any component of the drug.
Glaucoma, especially angle-closure.
Acute stage of myocardial infarction, especially with a decrease in blood pressure.
Acute circulatory failure, including cardiogenic shock, vascular collapse.
Arterial hypotension.
Concomitant use of molsidomine and phosphodiesterase-5 inhibitors, such as sildenafil, vardenafil, tadalafil (see section "Interaction with other medicinal products and other types of interactions").
The simultaneous administration of nitric oxide donors in any form and soluble guanylate cyclase stimulators (e.g. riociguat) is contraindicated due to the risk of arterial hypotension (see section "Interaction with other medicinal products and other types of interactions").
Pregnancy and breastfeeding.
Interaction with other medicinal products and other types of interactions
With simultaneous use of molsidomine with peripheral vasodilators, calcium ion antagonists, and antihypertensive agents, the hypotensive effect is potentiated.
When molsidomine is used simultaneously with acetylsalicylic acid, the antiplatelet effect is potentiated.
The combined use of molsidomine and iloprost leads to significant inhibition of platelet aggregation, therefore, if such a combination of drugs is necessary, appropriate studies should be performed to assess the blood picture and platelet aggregation.
When using the drug Molsicor, sildenafil should not be used due to the possibility of irreversible arterial hypotension with dangerous consequences.
The reason for this interaction is the mechanism of action of drugs for the treatment of erectile dysfunction containing phosphodiesterase-5 inhibitors as the active substance and molsidomine. Drugs for the treatment of erectile dysfunction containing phosphodiesterase-5 inhibitors as the active substance inhibit phosphodiesterase type 5 (PDE5), which is responsible for the metabolic breakdown of cyclic guanosine monophosphate (cGMP). At the same time, molsidomine is transformed, as a result of which cGMP is activated and blood vessels dilate. Thus, an increase in cGMP concentration due to the simultaneous administration of drugs for the treatment of erectile dysfunction containing phosphodiesterase-5 inhibitors as the active substance and molsidomine leads to a sudden decrease in blood pressure.
The drug can be used simultaneously with beta-blockers and calcium antagonists.
Alcohol enhances the effect of the drug, so drinking alcohol while using the drug is prohibited.
Ergot alkaloid. There is a potential pharmacodynamic interaction between nitric oxide donors and ergot alkaloids, which may lead to antagonistic drug effects. The concomitant use of nitric oxide donors and ergot alkaloids should be avoided.
The concomitant use of molsidomine and soluble guanylate cyclase stimulators (e.g. riociguat), which are nitric oxide receptor agonists, is contraindicated because the combination may lead to an increased risk of arterial hypotension.
Application features
Precautions and special precautions for use
Molsidomine does not usually cause a significant decrease in blood pressure, but caution should be exercised in patients with arterial hypotension, elderly patients with reduced circulating blood volume, and patients treated with other vasodilators. Careful monitoring should be ensured and the dose adjusted according to the patient's condition.
During therapy with Molsicor, it should be taken into account that resting blood pressure, particularly systolic blood pressure, may decrease. In this case, the hypertensive initial pressure reacts much more strongly than the normotensive or hypotensive pressure.
Use with caution in hypertrophic obstructive cardiomyopathy, constrictive (stenosing) pericarditis, pericardial tamponade, decreased pressure in the ventricles of the heart, aortic stenosis or mitral stenosis, acute myocardial infarction, impaired left ventricular function (left ventricular failure).
Special attention when treating with the drug is required for patients after hemorrhagic stroke, with cerebral circulation disorders and increased intracranial pressure; patients after a recent myocardial infarction, and patients with a tendency to hypotensive reactions.
With long-term use of nitrates, it is recommended to include molsidomine in the treatment regimen to prevent the development of nitrate tolerance.
Patients with hepatic or renal insufficiency should use lower doses with a gradual increase until the desired therapeutic effect is obtained. In severe liver dysfunction (increase in bromsulfalein test by 20-50%), the concentration of molsidomine in the blood plasma increases and the half-life increases, which requires dose adjustment of the drug.
It is generally not recommended to modify the dosage of molsidomine in the treatment of patients with impaired renal function. However, given that 90-95% of molsidomine metabolites are excreted by the kidneys, it is possible to reduce the dose or increase the intervals between doses of the drug, taking into account the individual patient's response to the drug.
Given that the drug contains lactose, it cannot be used in the treatment of patients with a rare form of congenital galactose intolerance, Lapp lactase deficiency or glucose-galactose malabsorption syndrome.
If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicine.
Due to the presence of the dye orange-yellow S (E 110) in the composition of the medicinal product, allergic reactions are possible.
Use during pregnancy or breastfeeding
Animal studies have not revealed any teratogenic effects of the drug. However, due to the lack of conclusive data on the safety of the drug, the use of Molsicor in the treatment of pregnant women is contraindicated.
Molsidomine passes into breast milk. The use of the drug is contraindicated during breastfeeding.
Ability to influence reaction speed when driving vehicles or other mechanisms
Given the adverse reactions of the drug (dizziness) and the possible negative impact on concentration in people who drive a car or work with other mechanisms, the drug can be prescribed with caution only after a thorough assessment of the possible risk.
Method of administration and doses
The dosage of the drug is set individually for each patient, depending on the degree of manifestations and phase of disease activity, as well as the patient's response to treatment.
Usually take 1 tablet 2 times a day (equivalent to 4 mg molsidomine/day). In some cases, it is sufficient to take 1/2 tablet 2 times a day (equivalent to 2 mg molsidomine/day). If necessary, the dose can be increased by 1-2 tablets 3 times a day (equivalent to 6-12 mg molsidomine/day) to the maximum daily dose - 2 tablets 4 times a day (equivalent to 16 mg molsidomine/day).
Special patient groups: In some patients, such as those with impaired hepatic or renal function, or those with congestive heart failure, or those treated with other vasodilators (see section 4.4), a lower initial dose may be appropriate.
The duration of treatment depends on the severity and course of the disease and is determined by the doctor.
Children.
Do not use the drug on children.
Overdose
Symptoms of overdose include severe headache, hypotension, tachycardia, bradycardia, weakness, drowsiness, dizziness, accompanied by nausea and vomiting, and in severe cases, states of collapse and shock.
Treatment: forced diuresis. In case of taking a dose significantly exceeding the usual single dose and if no more than an hour has passed, gastric lavage should be performed. If necessary, symptomatic treatment should be carried out. There is no data on the effectiveness of dialysis in case of overdose.
Side effects
The frequency of adverse reactions is classified as follows: very common (≥ 1/10), common (≥ 1/100, < 1/10), uncommon (≥ 1/1000, < 1/100), rare (≥ 1/10,000, < 1/1000), and frequency unknown.
Clinical trial data
On the part of the immune system
Rare: hypersensitivity reactions (e.g. cutaneous allergic reactions including skin rash, bronchospasm, asthma).
Central nervous system
Common: headache, which occurs at the beginning of treatment and usually disappears with continued treatment.
Rare: dizziness.
Frequency unknown: rapid fatigue, general weakness, slowed psychomotor and motor reactions (in most cases at the beginning of treatment).
Cardiovascular system
Common: hypotension. Treatment with Molsicor may lead to a decrease in blood pressure, rarely to the development of collapse or shock. In these cases, a dose reduction or discontinuation of the drug may be required.
Uncommon: reflex tachycardia, orthostatic dysregulation.
Frequency unknown: collapse.
From the digestive system
Rare: nausea.
Frequency unknown: loss of appetite, anorexia, vomiting, diarrhea.
From the skin side
Frequency unknown: facial flushing, urticaria.
Post-marketing surveillance data
During post-marketing surveillance for medicinal products containing molsidomine, the following adverse reactions have been observed:
Blood and lymphatic system disorders
Frequency unknown: thrombocytopenia.
On the part of the immune system
Very rare: anaphylactic shock.
Reporting adverse reactions after the registration of a medicinal product is important. This allows monitoring of the benefit/risk ratio when using this medicinal product. Medical and pharmaceutical professionals, as well as patients or their legal representatives, should report all cases of suspected adverse reactions and lack of efficacy of the medicinal product via the Automated Information System for Pharmacovigilance at the link: https://aisf.dec.gov.ua.
Expiration date
3 years.
Storage conditions
Store in the original packaging at a temperature not exceeding 25 °C, protected from light and out of the reach of children.
Packaging
30 tablets in a blister; 1 blister in a cardboard box.
Vacation category
According to the recipe.
Producer
Pharmaceutical Plant "POLPHARMA" S.A.
Location of the manufacturer and address of its place of business.
Metalowca Street 2, 39-460 Nowa Demba, Poland.
Applicant
PROPHARMA International Trading Limited, Malta.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.