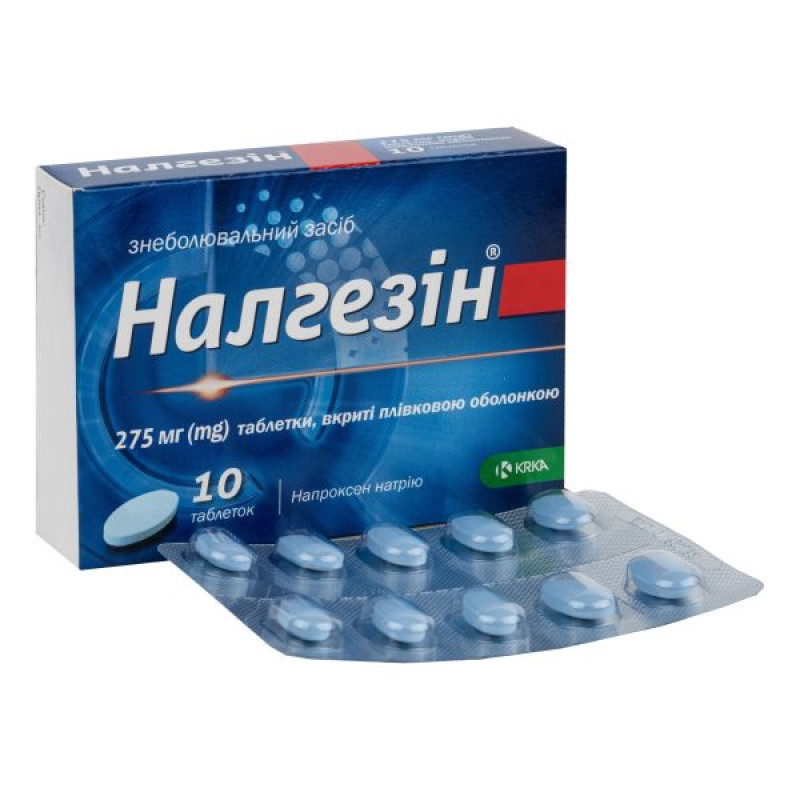
Nalgesin film-coated tablets 275 mg blister No. 10
Instructions for use Nalgesin film-coated tablets 275 mg blister No. 10
Composition
active ingredient: naproxen sodium;
1 film-coated tablet contains 275 mg of naproxen sodium;
Excipients: povidone, microcrystalline cellulose, talc, magnesium stearate, hypromellose, titanium dioxide (E 171), macrogol, indigo carmine (E 132), purified water.
Dosage form
Film-coated tablets.
Main physicochemical properties: oval, slightly biconvex tablets, film-coated, blue in color.
Pharmacotherapeutic group
Nonsteroidal anti-inflammatory and antirheumatic drugs. Propionic acid derivatives. Naproxen. ATC code M01A E02.
Pharmacological properties
Pharmacodynamics.
Naproxen sodium is a nonsteroidal anti-inflammatory drug. It has analgesic, anti-inflammatory and antipyretic effects. The mechanism of action of the drug is due to the inhibition of cyclooxygenase, an enzyme involved in the synthesis of prostaglandins. As a result, the levels of prostaglandins in various fluids and tissues of the body decrease.
Pharmacokinetics.
After oral administration, naproxen sodium is hydrolyzed in the acidic gastric juice. Naproxen microparticles are released, which then dissolve very quickly in the small intestine. This leads to a faster and more complete absorption of naproxen.
Peak plasma concentrations occur 1–2 hours after administration. Plasma levels of naproxen increase proportionally to dose up to 500 mg. At higher doses, they are less proportional. Approximately 99% of naproxen is bound to plasma albumin at drug concentrations up to 50 μg/mL.
Approximately 70% of naproxen is excreted unchanged and approximately 30% as the inactive metabolite 6-dimethyl-naproxen. Approximately 95% of the drug is excreted in the urine and 5% in the feces. The biological half-life of naproxen is independent of plasma concentration and dose and is 12–15 hours.
Indication
Nalgesin® is used:
for toothache and headache;
for pain in muscles, joints and spine;
to prevent and relieve migraines;
for menstrual pain.
Contraindication
Hypersensitivity to naproxen sodium or to any of the excipients.
Hypersensitivity to salicylates and other nonsteroidal anti-inflammatory drugs, manifested as bronchial asthma, urticaria, and rhinitis.
Acute period or recurrence of gastric or duodenal ulcer, gastrointestinal bleeding.
Severe liver and kidney dysfunction.
Heart failure.
Pregnancy and breastfeeding.
Age up to 16 years.
Interaction with other medicinal products and other types of interactions
Concomitant use of acetylsalicylic acid and other nonsteroidal anti-inflammatory drugs is not recommended due to the high risk of adverse reactions.
Clinical pharmacodynamic data suggest that concomitant use of naproxen for more than one consecutive day may inhibit the effect of low-dose acetylsalicylic acid on platelet activity, and this inhibition may persist for several days after discontinuation of naproxen therapy. The clinical significance of this interaction is unknown.
Concomitant use with antacids or cholestyramine, as well as with food, may slow down the absorption of naproxen, but does not affect the amount of active substance absorbed. Concomitant use with food may slow down the absorption of naproxen, but does not affect its amount.
Concomitant use with cardiac glycosides may lead to exacerbation of heart failure, decreased glomerular filtration rate, and increased levels of cardiac glycosides in the blood.
Naproxen should not be used within 8–12 days after taking mifepristone due to its ability to reduce the effects of the latter.
Caution should be exercised when using naproxen with corticosteroids due to the increased risk of gastrointestinal ulcers and bleeding.
Naproxen sodium may reduce platelet aggregation and prolong bleeding time, this should be taken into account when determining bleeding time and during concomitant treatment with anticoagulants.
Concomitant use of Naprosyn is not recommended due to its content of the same active ingredient, namely naproxen.
Animal studies have shown the potential for quinolone antibiotics to cause seizures. Patients taking quinolones are at increased risk of developing seizures.
Since naproxen is almost completely bound to plasma proteins, it should be used with caution when administered concomitantly with hydantoin derivatives and sulfonylurea derivatives.
Naproxen may reduce the natriuretic effect of furosemide.
Naproxen may reduce the effect of antihypertensive drugs.
With concomitant administration of lithium and naproxen sodium, the concentration of lithium in the blood plasma increases.
Naproxen, like other NSAIDs, may reduce the antihypertensive effect of propranolol and other beta-blockers and increase the risk of renal failure in patients taking ACE inhibitors concomitantly.
Concomitant administration of probenecid prolongs the biological half-life of naproxen and increases its plasma concentration.
Concomitant use of cyclosporine may increase the risk of renal impairment.
In vitro studies have shown that concomitant administration of naproxen sodium and zidovudine increases plasma concentrations of the latter.
Application features
Minimization of adverse reactions can be achieved by using the lowest effective doses and reducing the duration of Nalgesin® administration.
In the presence of an infectious disease, the anti-inflammatory and antipyretic effects of naproxen should be taken into account, which may mask the signs of these diseases.
Naproxen and its metabolites are primarily eliminated by the kidneys by glomerular filtration, so naproxen sodium should be administered with great caution to patients with impaired renal function. In patients with renal insufficiency, creatinine clearance should be determined and monitored during treatment. Naproxen is not recommended if creatinine clearance is less than 20 ml/min (0.33 ml/s).
Special caution should be exercised in patients with impaired liver function. In chronic alcoholic cirrhosis of the liver and possibly other forms of cirrhosis, the total plasma concentration of naproxen sodium is reduced and the plasma concentration of unbound naproxen sodium is increased. It is recommended to take the lowest effective dose.
Physicians should closely monitor patients with epilepsy or porphyria who are taking naproxen.
Elderly patients should take Nalgesin® in the lowest effective doses.
Patients with gastrointestinal diseases, especially ulcerative colitis or Crohn's disease (also in the past), who take naproxen sodium need close medical supervision due to possible relapse or exacerbation of the disease. Serious adverse events related to the gastrointestinal tract may occur in the absence of such problems in the past.
As with other NSAIDs, the overall incidence of serious adverse events, gastrointestinal bleeding and perforation, increases linearly with duration of treatment. Taking higher doses of naproxen may also increase the risk of adverse events.
In case of prolonged use, constant supervision is necessary to detect adverse reactions. Elderly and debilitated patients are more prone to the formation of gastrointestinal ulcers, bleeding and the development of serious adverse reactions. In persons with a history of bronchial asthma, allergic diseases or cases of bronchospasm, bronchospasm may develop. Deviations in the results of laboratory tests of liver function are possible. Naproxen reduces platelet aggregation and prolongs bleeding time. This should be taken into account when determining bleeding time. When using naproxen, slight peripheral edema is possible, the risk of its development is greater in patients with impaired cardiac function. Patients with impaired blood clotting and patients taking drugs that affect hemostasis require special supervision. In case of simultaneous use of anticoagulants, the risk of bleeding increases.
Anaphylactoid (anaphylactic) reactions may occur in individuals with or without a history of hypersensitivity reactions to aspirin, other NSAIDs, or naproxen-containing products. Patients with a history of angioedema, bronchospasm, asthma, rhinitis, and nasal polyps may also develop anaphylactoid reactions. Some of these reactions, such as anaphylactic shock, may be fatal.
If the dose of steroid medications is reduced or discontinued during naproxen therapy, the dose should be reduced gradually and under close medical supervision to detect any adverse reactions, including adrenal insufficiency and exacerbation of arthritis symptoms.
Ocular disorders, including papillitis, retrobulbar neuritis, and papilledema, have been reported rarely with NSAIDs, including naproxen, although a causal relationship has not been established. Therefore, patients who develop visual disturbances while taking naproxen should have an ophthalmological examination.
Before starting treatment with naproxen, it is necessary to clarify (discuss with a doctor) the presence in the patient's medical history of hypertension and/or heart failure with fluid retention and edema associated with taking NSAIDs.
Clinical trial and epidemiological data suggest that an increased risk of arterial thrombosis may be associated with the use of some NSAIDs (especially at high doses and over long periods). According to these data, naproxen (1000 mg daily) is associated with a lower risk, but risks cannot be completely excluded.
The use of naproxen should be carefully considered in patients with uncontrolled hypertension, congestive heart failure, ischemic heart disease, peripheral arterial disease, and/or cerebrovascular disease. In individuals with risk factors for cardiovascular events (e.g., hypertension, hyperlipidemia, diabetes, smoking), the use of naproxen should also be carefully considered before initiating long-term therapy.
Naproxen, like other cyclooxygenase inhibitors, may affect fertility. Women who are trying to conceive and/or have difficulty conceiving should discontinue naproxen.
Important information about some of the ingredients of Nalgesin®
This medicinal product contains 1.09 mmol (25.097 mg) sodium per dose. This should be taken into consideration by patients on a controlled sodium diet.
Use during pregnancy or breastfeeding
The use of Nalgesin® during pregnancy is contraindicated. From the 20th week of pregnancy onwards, the use of Nalgesin® may cause oligohydramnios due to fetal renal dysfunction. This disorder may occur shortly after initiation of treatment and is usually reversible upon discontinuation of treatment. It may be advisable to monitor prenatally for oligohydramnios and narrowing of the ductus arteriosus after exposure to Nalgesin® for several days, starting from the 20th week of gestation. The use of Nalgesin® should be discontinued if oligohydramnios or narrowing of the ductus arteriosus is detected.
If it is necessary to use Nalgesin® during lactation, breastfeeding should be discontinued.
Ability to influence reaction speed when driving vehicles or other mechanisms
While using Nalgesin®, some patients may experience drowsiness, dizziness, vertigo, visual disturbances, insomnia, or depression, which may affect the speed of reaction when driving vehicles and operating other mechanisms.
Method of administration and doses
The tablets should be swallowed whole with a glass of water. Treatment should be started with the lowest recommended dose.
Adults and children aged 16 and over
Toothache, headache, muscle, joint and spine pain
The recommended dose is 2 tablets (550 mg) 2 times a day, but not more than 4 tablets (1100 mg) per day. Except for severe pain (except for musculoskeletal diseases), in which case the dose can be increased to 5 tablets per day (1375 mg).
Migraine
At the first signs of an attack, the recommended initial dose is 3 tablets (825 mg). If necessary, 1 tablet (275 mg) or 2 tablets (550 mg) can be taken additionally, but not earlier than half an hour after taking the initial dose. The daily dose should not exceed 5 tablets (1375 mg).
Menstrual pain
The recommended starting dose is 2 tablets (550 mg), if necessary, you can take 1 tablet (275 mg) every 6-8 hours. The maximum dose is 5 tablets (1375 mg) on the first day of taking and 4 tablets (1100 mg) on the following days.
The duration of treatment for pain relief is 10 days. If symptoms persist, consult a doctor.
Elderly patients
Nalgesin® should be used in the lowest effective doses.
Patients over 65 years of age should take tablets no more frequently than every 12 hours, if necessary.
Patients with renal impairment
Nalgesin® should be used in the lowest effective doses.
Patients with hepatic impairment
Nalgesin® should be used in the lowest effective doses.
Minimization of adverse reactions can be achieved by using the lowest effective doses and reducing the duration of Nalgesin® administration.
Children.
Nalgesin® is contraindicated in children under 16 years of age.
Overdose
After accidental or intentional ingestion of large amounts of naproxen sodium, abdominal pain, nausea, vomiting, dizziness, tinnitus, irritability, and in more severe cases, hematemesis, melena, impaired consciousness, respiratory distress, convulsions, and renal failure may occur. The following treatment is indicated: gastric lavage, activated charcoal, antacids, H2-receptor inhibitors, proton pump inhibitors, misoprostol, and other forms of symptomatic treatment.
Side effects
From the side of the hematopoietic and lymphatic systems: eosinophilia, granulocytopenia, leukopenia, thrombocytopenia, agranulocytosis, aplastic anemia, hemolytic anemia.
Immune system disorders: hypersensitivity reactions, anaphylactic reactions, angioedema.
Psychiatric: convulsions, abnormal dreams.
Nervous system: headache, vertigo, dizziness, drowsiness, depression, sleep disturbances, inability to concentrate, insomnia, weakness, aseptic meningitis, cognitive disorders.
On the part of the organs of vision: visual impairment, corneal opacity, papillitis, retrobulbar neuritis, swelling of the optic nerve papilla.
Patients who develop visual disturbances during treatment with naproxen should undergo an ophthalmological examination.
From the side of the organs of hearing and labyrinth: tinnitus, hearing impairment, hearing impairment.
Cardiac: edema, palpitations, congestive heart failure.
Respiratory, thoracic and mediastinal disorders: dyspnea, eosinophilic pneumonia, asthma, pulmonary edema.
Metabolism and nutrition disorders: hyperkalemia, hypokalemia.
Gastrointestinal: constipation, abdominal pain, nausea, dyspepsia, diarrhea, stomatitis, gastrointestinal bleeding and/or gastric perforation, hematemesis, melena, vomiting, ulcerative stomatitis, colitis, esophagitis, pancreatitis, peptic ulcer formation.
Liver and biliary tract: increased liver enzymes, jaundice, hepatitis.
Musculoskeletal and connective tissue disorders: muscle pain and muscle weakness.
Renal and urinary disorders: glomerulonephritis, hematuria, interstitial nephritis, nephrotic syndrome, renal dysfunction, renal failure, renal papillary necrosis.
Reproductive system and breast disorders: infertility in women.
Skin and subcutaneous tissue disorders: pruritus, skin rash, bruising, purpura, alopecia, photosensitive dermatitis, erythema nodosum, lichen planus, pustules, systemic lupus erythematosus, epidermal necrolysis, erythema multiforme, photosensitivity reactions similar to chronic hematoporphyria and bullous epidermolysis, Stevens-Johnson syndrome, urticaria.
General disorders and administration site conditions: thirst, sweating, menstrual irregularities, hyperthermia (chills and fever).
Impact on laboratory and instrumental test results: increased creatinine levels.
Edema, hypertension, and heart failure have been reported in association with NSAIDs.
Clinical trial results and epidemiological data suggest that an increased risk of arterial thrombotic events (e.g. myocardial infarction or stroke) may be associated with the use of some NSAIDs (especially at high doses and over long periods of time).
If severe adverse reactions occur, treatment should be discontinued.
Expiration date
5 years.
Storage conditions
This medicinal product does not require any special storage conditions. Keep the blister in the outer carton in order to protect from light. Keep out of the reach of children.
Packaging
10 tablets in a blister; 1 or 2 blisters in a cardboard box.
Vacation category
Without a prescription.
Producer
KRKA, dd, Novo mesto/ KRKA, dd, Novo mesto.
Address
Smarjeska cesta 6, 8501 Novo mesto, Slovenia.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.