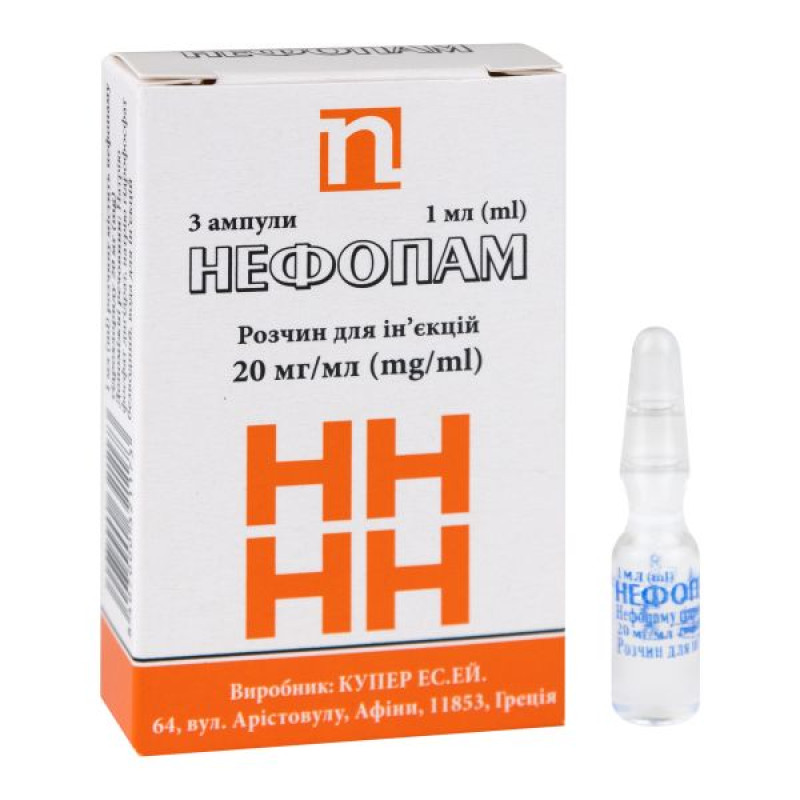
Nefopam solution for injection 20 mg/ml ampoule 1 ml No. 3

Instructions Nefopam solution for injection 20 mg/ml ampoule 1 ml No. 3
Composition
active ingredient: nefopam;
1 ml of solution contains nefopam hydrochloride 20 mg;
Excipients: sodium phosphate dihydrate, sodium hydrogen phosphate anhydrous, water for injections.
Dosage form
Solution for injection.
Main physicochemical properties: transparent colorless solution.
Pharmacotherapeutic group
Analgesics and antipyretics. ATX code N02B G06.
Pharmacological properties
Pharmacodynamics
Nefopam is a non-narcotic analgesic, structurally unlike other analgesics. Experimental studies indicate a central action consisting in the inhibition of the reuptake of dopamine, noradrenaline and serotonin at the synapse level. In animals, nefopam has shown antinociceptive activity through a possible reduction in the release of glutamate at the presynaptic level and the activation of N-methyl-D-aspartate receptors at the postsynaptic level. In clinical studies, nefopam has shown a positive effect on postoperative tremor. Nefopam does not have anti-inflammatory or antipyretic effects, does not depress respiration and does not affect intestinal peristalsis. Nefopam has a slight anticholinergic effect.
Pharmacokinetics
After a single dose of 20 mg intramuscularly, the peak serum concentration is observed after 30-60 minutes, and the maximum concentration is 25 ng/ml. The half-life is on average 5 hours. After intravenous administration of a dose of 20 mg, the half-life is 4 hours. Plasma protein binding is 71-76%. Biotransformation is significant, three metabolites have been identified: desmethylnefopam; nefopam N-oxide; nefopam N-glucuronide.
Desmethylnefopam and nefopam N-oxide are unconjugated and do not exhibit analgesic activity in animal studies. 87% of the administered dose is excreted by the kidneys, less than 5% of the administered dose is excreted unchanged.
Metabolites detected in urine account for 6%, 3% and 36%, respectively, of the intravenously administered dose.
Indication
Postoperative analgesia as part of multimodal analgesia (nefopam also has positive properties in preventing postoperative tremor).
Pain syndrome of various etiologies and intensity (injuries, pain after surgical operations, childbirth anesthesia, toothache, myalgia, renal and hepatic colic). Premedication before painful medical procedures.
Contraindication
Hypersensitivity to nefopam or to any of the other ingredients of the drug. Convulsions or history of them. Risk of urinary retention associated with urethroprostatic disorders. Risk of acute glaucomatous attack. Concomitant use of MAO inhibitors.
Interaction with other medicinal products and other types of interactions
It should be taken into account that a significant number of medications can increase the depression of the nervous system due to an additive effect and can reduce alertness.
Drugs affected: opiates (analgesics, antitussives, substitution drugs for the treatment of drug addiction), neuroleptics, barbiturates, benzodiazepines, non-benzodiazepine tranquilizers (meprobamate), hypnotics, antidepressants with sedative effects (amitriptyline, doxepin, mianserin, mirtazapine, trimipramine), sedative H1-histamine receptor blockers, centrally acting antihypertensives, baclofen, thalidomide.
Application features
Nefopam is not a morphine-like drug or an opiate antagonist. Therefore, discontinuation of morphine-like drug treatment in morphine-dependent patients already taking Nefopam increases the risk of withdrawal syndrome. Furthermore, Nefopam does not enhance detoxification in patients.
The risk/benefit ratio of Nefopam treatment must be constantly assessed.
Nefopam should not be prescribed for the treatment of chronic pain syndromes such as headache.
Caution should be exercised when prescribing the drug to patients with hepatic or renal insufficiency, and to elderly patients.
Caution should be exercised when prescribing to patients with tachycardia.
There is a possible risk of pharmacological dependence in patients with depression or in patients with a history of any pharmacological dependence.
The use of alcohol and medications containing alcohol should be avoided due to the increased sedative effect when consumed with alcohol.
Do not use in patients for the treatment of myocardial infarction.
Use during pregnancy or breastfeeding
The drug is not used during pregnancy or breastfeeding.
Ability to influence reaction speed when driving vehicles or other mechanisms
The possible risk of drowsiness during treatment with the drug should be taken into account, which may affect the ability to drive or operate other mechanisms.
Method of administration and doses
Intravenous administration. Nefopam should be administered as a continuous intravenous infusion at a rate of not more than 5 mg/min for at least 15 minutes, the patient should be in a supine position. A single dose per injection is 20 mg. If necessary, the injection is repeated every 4 hours. The maximum daily dose is 120 mg.
Input method.
Nefopam can be administered in a standard infusion solution (0.9% sodium chloride solution or 5% glucose solution). The optimal dilution ratio is 1 ampoule of the drug in 50 ml of infusion solution.
The course of treatment is no more than 8-10 days.
Children
Do not use in children under 15 years of age.
Overdose
Symptoms: tachycardia, convulsions, hallucinations, agitation.
Treatment: symptomatic treatment, cardiological and respiratory monitoring in a hospital setting.
Adverse reactions
Central nervous system: drowsiness, dizziness*, syncope, convulsions*, tremor, blurred vision, insomnia, headache, paresthesia.
Psychiatric: agitation*, irritability*, hallucinations, drug dependence, confusion.
Cardiac: tachycardia*, hypotension, palpitations*.
Gastrointestinal: nausea, vomiting, dry mouth*, abdominal pain, diarrhea.
From the urinary system: rarely, urine color change, urinary retention may occur.
Immune system disorders: hypersensitivity reactions, including urticaria, angioedema, anaphylactic shock.
General disorders: hyperhidrosis*, malaise, injection site changes.
*Other atropine-like reactions are rare, as nefopam has a slight anticholinergic effect.
Expiration date
3 years.
Storage conditions
Store in a dry place at a temperature of 15-25 °C.
Avoid freezing.
Keep out of reach of children.
Incompatibility
Do not mix the drug with other medicines in the same container.
Packaging
1 ml in an ampoule, 3 ampoules in a cassette, 1 cassette in a cardboard box.
Vacation category
According to the recipe.
Producer
Pharma Mediterrania, S.L.
Location of the manufacturer and its business address
San Sebastián s/n, Sant Just Desvern, 08960 (Barcelona), Spain.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.