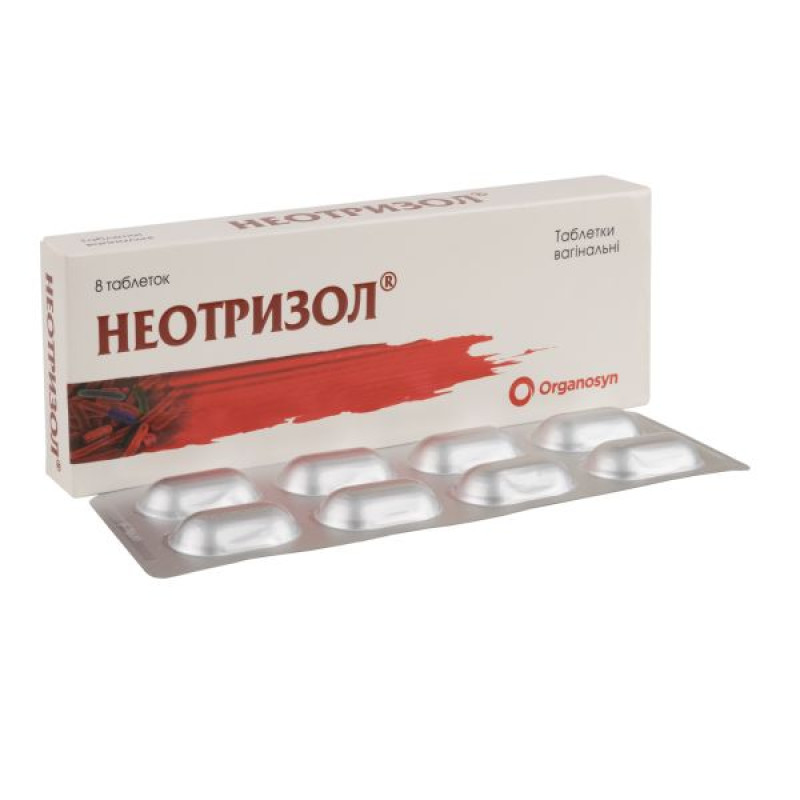
Neotrizol vaginal tablets blister with applicator No. 8
Instructions Neotrizol vaginal tablets blister with applicator No. 8
Composition
active ingredients: ornidazole, neomycin, miconazole, prednisolone;
1 vaginal tablet contains ornidazole 500 mg, neomycin sulfate 100 mg (equivalent to 68,000 IU of activity), miconazole nitrate 100 mg, prednisolone 3 mg;
Excipients: lactose monohydrate, povidone K-30, corn starch, talc, colloidal anhydrous silica, sodium starch glycolate (type A), magnesium stearate, croscarmellose sodium.
Dosage form
Vaginal tablets.
Main physicochemical properties: oblong, biconvex tablets without a shell, almost white in color, with a rounded edge on one side and flat on the other.
Pharmacotherapeutic group
Antimicrobial/antiseptic agents in combination with corticosteroids. Imidazole derivatives and corticosteroids. ATX code G01B F.
Pharmacological properties
Pharmacodynamics
Ornidazole. The mechanism of action of ornidazole is associated with disruption of the DNA structure in microorganisms sensitive to it. The drug easily penetrates the microbial cell and, binding to DNA, disrupts the replication process. Ornidazole is active against Trichomonas vaginalis, Entamoeba histolytica, Giardia lamblia (Giardia intestinalis), as well as some anaerobic bacteria, such as Bacteroides and Clostridium spp., Fusobacterium spp. and anaerobic cocci.
Miconazole. Miconazole is an antifungal drug that is intended for the topical treatment of vulvovaginal candidiasis (moniliasis), active only against candidal vulvovaginatis.
Neomycin. Neomycin is an antibiotic belonging to the aminoglycoside group. Aminoglycosides have high activity against gram-negative and are also effective against gram-positive bacteria. Penetrates the structure of the bacterial cell by producing abnormal proteins. These proteins block the synthesis of proteins necessary for the survival of bacteria.
Prednisolone. Prednisolone is a synthetic corticosteroid drug used to reduce the symptoms of inflammation. Prednisolone inhibits the secretion and release of inflammatory mediators and proliferative processes in inflammatory diseases and reduces the likelihood of scarring.
Pharmacokinetics
Despite the topical application of the drug, systemic absorption is possible, especially in case of inflammation of the vagina. Systemic effects of the components are not expected, but slight systemic absorption should be considered.
The concentration of ornidazole in vaginal tissues significantly exceeds the concentration achieved with its oral and intravenous administration.
Systemic absorption of miconazole is limited when applied topically. It has selective toxicity for yeast-like fungi of the genus Candida.
Neomycin hardly penetrates the body through intact skin. It penetrates the structure of the bacterial cell by producing abnormal proteins. These proteins block the synthesis of proteins necessary for the survival of the bacteria.
Prednisolone reduces the symptoms of inflammation, including hyperemia.
Indication
Treatment of combined gynecological diseases, including bacterial vaginosis and vaginitis (caused by Candida albicans), mixed infections (caused by Trichomonas, anaerobic infection, including Gardnerella and yeast-like fungi).
Prevention of gynecological diseases before surgical treatment.
Vaginal sanitation: before childbirth or abortion, before and after the introduction of intrauterine contraceptives, before and after diathermocoagulation of cervical erosions, before intrauterine examinations.
Contraindication
Hypersensitivity to the active substances, to other imidazole derivatives and to other components of the drug.
Systemic and local infectious diseases, until specific therapy is prescribed.
Interaction with other medicinal products and other types of interactions
No clinically significant drug interactions have been identified. However, the possible systemic absorption of the drug should be taken into account, especially in the presence of inflammation.
Interactions related to prednisolone
Undesirable combinations:
with acetylsalicylic acid: increased risk of bleeding when used simultaneously with anti-inflammatory doses of acetylsalicylic acid (≥ 1 g per dose or ≥ 3 g per day).
Combinations that require careful use:
with anticonvulsants, enzyme inducers: decreased plasma concentrations and efficacy of corticosteroids by increasing their metabolism in the liver. The consequences of this are particularly severe (or important) for patients with Addison's disease treated with hydrocortisone and in the case of organ transplantation. Monitoring of clinical and laboratory tests is necessary, as well as corticosteroid dose adjustment during therapy and after discontinuation of treatment with enzyme inducers;
with rifampicin: decreased plasma concentrations and efficacy of corticosteroids by increased hepatic metabolism following interaction with rifampicin. The consequences are particularly severe (or important) in patients with Addison's disease treated with hydrocortisone and in organ transplant recipients. Clinical and laboratory monitoring and corticosteroid dose adjustment are necessary during and after rifampicin therapy;
with other hypokalemic drugs: increased risk of hypokalemia. Serum potassium levels should be monitored and adjusted if necessary;
with digitalis preparations: hypokalemia exacerbates the toxic effects of digitalis preparations. Before treatment with the drug, assess for hypokalemia, determine serum potassium levels, and perform electrocardiography;
with drugs that cause torsades de pointes: increased risk of torsades de pointes, including torsades de pointes. Before treatment with the drug, assess for hypokalemia, determine serum potassium levels, and perform electrocardiography.
Combinations that require attention:
with cyclosporine: increased effects of prednisolone, including Cushingoid states, decreased carbohydrate tolerance (decreased clearance of prednisolone);
with acetylsalicylic acid: increased risk of bleeding with antipyretic or analgesic doses ≥ 500 mg per dose or < 3 g per day;
with nonsteroidal anti-inflammatory drugs: increased risk of peptic ulcer and gastrointestinal bleeding;
with fluoroquinolones: there may be an increased risk of tendinitis or even tendon rupture (rare), particularly in patients who have been treated with corticosteroids for a long time.
Application features
Simultaneous treatment of the sexual partner is necessary to prevent the risk of re-infection.
The drug should be used with caution under constant medical supervision by specialists in patients who are simultaneously using systemic corticosteroids, with osteoporosis, arterial hypertension, heart failure, with existing or history of serious emotional disorders, tuberculosis, diabetes mellitus, glaucoma, with myopathy caused by the use of corticosteroids, liver failure, epilepsy, peptic ulcer, hypothyroidism, and recent myocardial infarction.
The drug should be used with caution in patients with severe pseudoparalytic myasthenia gravis, nonspecific colitis, diverticulitis, and fresh intestinal anastomoses.
Despite the topical application of the drug, systemic absorption is possible, especially in case of vaginal inflammation.
Despite topical application, slight absorption of various components is possible (see "Adverse reactions").
Hypersensitivity to topical application may indicate that the use of these or related antibiotics is undesirable.
Precautions during use.
The duration of treatment should be limited to reduce the risk of the emergence of resistant microorganisms or superinfection caused by these microorganisms.
The drug contains lactose, therefore patients with rare hereditary forms of galactose intolerance, lactase deficiency or glucose-galactose malabsorption syndrome should not use Neotrizol®.
Use during pregnancy or breastfeeding
When deciding whether to use the drug in pregnant women, the benefit/risk ratio should be weighed. Since corticosteroids may pass into breast milk in small amounts, possible signs of adrenal suppression should be monitored.
Ability to influence reaction speed when driving vehicles or other mechanisms
No data.
Method of administration and doses
The drug is administered deep into the vagina, 1 tablet at night. After administration, lie down for at least 15 minutes. Treatment should not be interrupted during menstruation. Tablets should be continued even when all symptoms of the disease have disappeared.
The average duration of treatment and prophylaxis with the drug is 8 days. If after an 8-day course of therapy the symptoms have not disappeared, the treatment should be repeated.
Entry procedure
Place the vaginal tablet in the applicator.
Immerse the applicator with the tablet in warm (30–40 °C) boiled water for a few seconds.
Carefully insert the applicator with the tablet as deep as possible into the vagina (preferably while lying on your back).
Leave the tablet in the vagina, removing it from the applicator, then wash the applicator with warm soapy water, rinse and dry.
Children
The drug should not be used in children.
Overdose
Due to the local effect of the drug, overdose has not been observed, but increased manifestations of adverse reactions are possible. Treatment is symptomatic.
Side effects
With topical application, slight absorption of the drug components is possible with the development of systemic effects.
On the part of the immune system: hypersensitivity, allergic reactions, including allergic dermatitis, allergic stomatitis, itching, rash, urticaria.
If hypersensitivity reactions or irritation develop, discontinue use of the drug.
Skin and subcutaneous tissue disorders: delayed healing of wounds and cracks.
Reproductive system and breast disorders: application site reactions, including irritation, itching, cramps, burning, pain, erosions, mucosal atrophy.
Expiration date
2 years.
Storage conditions
Store in the original packaging out of the reach of children at a temperature not exceeding 25 °C.
Packaging
4 tablets in a strip; 2 strips in a cardboard box, complete with an applicator.
8 tablets in a blister; 1 blister in a cardboard box, complete with an applicator.
Vacation category
According to the recipe.
Producer
Evertogen Life Sciences Limited.
Location of the manufacturer and address of its place of business.
Plot No.: S-8, S-9, S-13/P and S-14/P T E C I A C, S I Z Pharma, Green Industrial Park, Polepally (V), Yedcherla (M), Mahabubnagar, Telangana, IH-509 301, India /
Plot No: S-8, S-9, S-13/P & S-14/P TSIIC, Pharma SEZ, Green Industrial Park, Polepally (V), Jadcherla (M), Mahabubnagar, Telangana, IN-509 301, India.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.