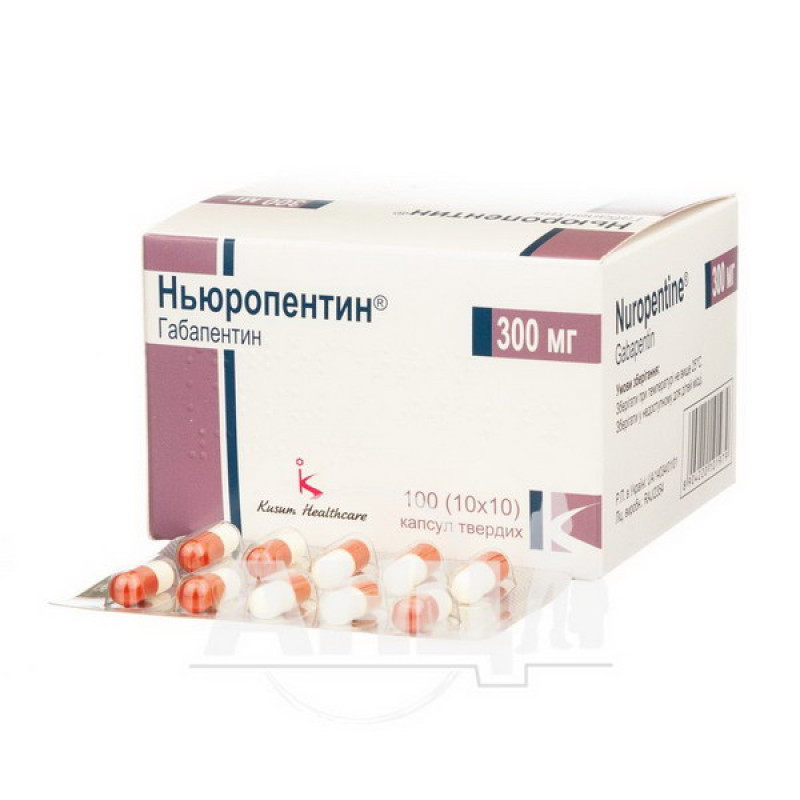
Neuropentin hard capsules 300 mg blister No. 100

Instructions Neuropentin hard capsules 300 mg blister No. 100
Composition
active ingredient: gabapentin;
1 hard capsule contains 300 mg of gabapentin;
excipients: mannitol (E 421), corn starch, magnesium stearate, talc, colloidal anhydrous silicon dioxide; hard gelatin capsule: gelatin, purified water, red iron oxide (E 172), titanium dioxide (E 171).
Dosage form
The capsules are hard.
Main physicochemical properties: hard gelatin capsules size 1 with a white body and a red cap, containing a white or almost white powder.
Pharmacotherapeutic group
Antiepileptic drugs. ATX code N0ZA X12.
Pharmacological properties
Pharmacodynamics.
Gabapentin – 1-(aminomethyl)-cyclohexanoacetic acid – is a cyclic analogue of gamma-aminobutyric acid (GABA), which can penetrate the blood-brain barrier. The anticonvulsant activity of gabapentin has been shown in many experimental models of convulsive states. The mechanism of the antiepileptic action of gabapentin is currently unknown. Despite the fact that gabapentin is structurally similar to GABA, it is not a GABA mimetic, since it does not bind to either GABAA or GABAB receptors, does not inhibit GABA reuptake or GABA degradation with the participation of GABA transaminase. It does not interact with voltage-gated sodium channels, benzodiazepine receptors, binding sites of excitatory neurotransmitters, and does not affect catecholamine, acetylcholine, or opiate receptors. Thus, gabapentin has a completely new mechanism of action, binding to highly specific centers in the central nervous system (CNS), which are proteinaceous in nature, localized mainly in the neocortex, and have no affinity for other antiepileptic drugs.
Gabapentin is also effective in relieving neuropathic pain.
Pharmacokinetics.
Absorption is rapid. Bioavailability is approximately 60%. Bioavailability is not dose-proportional: with increasing dose, bioavailability decreases and is 60% at a dose of 300 mg, and 30% at a dose of 1600 mg. Food does not affect the pharmacokinetics of gabapentin. The time to reach maximum concentration is 2–3 hours. The concentration of the drug in blood plasma is proportional to the dose. Pharmacokinetics do not change with repeated administration. Passes through the blood-brain barrier: in patients with epilepsy, the concentration of gabapentin in the cerebrospinal fluid is approximately 20% of the corresponding equilibrium concentration of the drug in blood plasma. Passes into breast milk. Gabapentin does not bind to plasma proteins, the volume of distribution is 57.7 liters. Gabapentin is practically not metabolized. Does not induce liver oxidative enzymes. Excreted by the kidneys unchanged. The half-life is independent of dose and averages 5–7 hours in patients with normal renal function. In elderly patients and in patients with impaired renal function, the rate of excretion decreases in direct proportion to the level of creatinine clearance. It is removed from the blood by hemodialysis. Dose adjustment is recommended for patients with impaired renal function and patients on hemodialysis.
The pharmacokinetics of gabapentin in children were evaluated in 50 healthy subjects aged 1 month to 12 years. Overall, when calculated on a dose per kilogram of body weight (mg/kg), plasma concentrations of gabapentin in children aged 5 years and older did not differ from those in adults.
Indication
Epilepsy.
Gabapentin is used as an adjunctive drug in the treatment of partial seizures with or without secondary generalization in adults and children aged 6 years and older.
Gabapentin is used as monotherapy for partial seizures with or without secondary generalization in adults and children aged 12 years and older.
Neuropathic pain.
Gabapentin is indicated for the treatment of peripheral neuropathic pain, such as painful diabetic neuropathy and postherpetic neuralgia, in adults.
Contraindication
Hypersensitivity to the active substance or to any of the excipients.
Interaction with other medicinal products and other types of interactions
CNS depressants (including opioids)
There are reports from various sources (spontaneous reports and literature) of cases of respiratory depression, sedation and death when gabapentin is used concomitantly with CNS depressants, including opioids. These complications have been reported to occur predominantly when gabapentin is used concomitantly with opioids in debilitated patients, as well as in elderly patients or those with serious underlying respiratory disease, polypharmacy or substance abuse.
Morphine
In clinical studies, concomitant administration of gabapentin and morphine resulted in a 44% increase in gabapentin AUC. Therefore, patients receiving these drugs should be closely monitored for signs of CNS depression, including drowsiness, sedation, and respiratory depression. If these occur, the dose of gabapentin or morphine should be appropriately reduced.
No interactions between these drugs and gabapentin were observed.
The steady-state pharmacokinetics of gabapentin in plasma are similar in healthy volunteers and in patients with epilepsy receiving these antiepileptic drugs.
Oral contraceptives containing norethindrone and/or ethinyl estradiol: Co-administration with gabapentin does not affect the pharmacokinetics of the drugs.
Antacid medicines containing aluminum and magnesium
Concomitant administration of these drugs with gabapentin results in a 24% decrease in gabapentin bioavailability. Gabapentin should be taken no earlier than 2 hours after taking an antacid.
Probenecid does not affect the renal excretion of gabapentin.
Cimetidine
Concomitant administration of this drug with gabapentin leads to a slight decrease in the renal excretion of gabapentin, which is not clinically significant.
Application features
Severe cutaneous adverse reactions (SCARs)
Severe cutaneous adverse reactions (SCARs), including Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug rash with eosinophilia and systemic symptoms (DRESS), which can be life-threatening or fatal, have been reported in association with gabapentin treatment. Patients should be advised of the signs and symptoms and monitored closely for skin reactions when prescribing the drug. If signs and symptoms suggestive of these reactions occur, gabapentin should be discontinued immediately and alternative treatment considered (if appropriate).
If a patient has developed a serious reaction such as SJS, TEN or DRESS syndrome with gabapentin, gabapentin treatment should never be resumed.
Anaphylaxis
Gabapentin may cause anaphylaxis. The reported cases of anaphylaxis have included difficulty breathing, swelling (lips, throat, and tongue), hypotension, and required emergency medical attention. Patients should be advised to discontinue gabapentin and seek immediate medical attention if any signs of anaphylaxis occur.
Suicidal thoughts and behavior.
Suicidal ideation and behavior have been observed in patients treated with antiepileptic drugs in some indications. A meta-analysis of randomized placebo-controlled trials of antiepileptic drugs also showed a small increased risk of suicidal ideation and behavior, the mechanism of which is unknown. In the post-marketing period, cases of suicidal ideation/behavior have been observed in patients treated with gabapentin (see section "Adverse reactions").
Patients (and caregivers of patients) should be advised to seek medical advice if signs of suicidal ideation and behavior emerge. Patients taking gabapentin should be monitored for signs of suicidal ideation and behavior and appropriate treatment should be initiated as necessary. Discontinuation of gabapentin should be considered if suicidal ideation/behavior emerges.
Acute pancreatitis
If symptoms of pancreatitis appear while taking gabapentin, the drug should be discontinued.
Convulsions.
Although there is no evidence of recurrence of seizures after discontinuation of gabapentin, abrupt discontinuation of anticonvulsant therapy in patients with epilepsy may precipitate status epilepticus (see section 4.2).
As with other antiepileptic drugs, some patients may experience an increase in seizure frequency or the development of new seizure types when taking gabapentin.
Attempts to discontinue concomitant antiepileptic drugs in order to switch to gabapentin monotherapy in patients receiving multiple antiepileptic drugs have rarely been successful.
Gabapentin is not considered effective in the treatment of primary generalized seizures such as absences and may increase the intensity of such seizures in some patients. For this reason, gabapentin should be used with caution in patients with mixed seizures including absences.
Gabapentin treatment has been associated with dizziness and somnolence, which could potentially increase the risk of accidental injury (falls). Loss of consciousness, confusion, and psychiatric disorders have also been reported. Therefore, patients should be advised to exercise caution until their individual response to gabapentin is known.
Concomitant use with opioids and other CNS depressants
Caution is advised when gabapentin is co-administered with opioids due to the risk of CNS depression. In a population-based, observational case-control study of patients taking opioids, co-administration with gabapentin was associated with an increased risk of opioid-related death compared with opioid use alone (adjusted odds ratio (aOR) 1.49 [95% CI, 1.18–1.88, p< 0.001]).
Respiratory depression
Gabapentin treatment has been associated with severe respiratory depression. Patients with respiratory disorders, respiratory or neurological diseases, renal impairment, concomitant use of CNS depressants, and the elderly are at increased risk of this severe adverse reaction. Dose adjustment may be necessary in such patients.
Elderly patients (over 65 years of age)
There have been no systematic studies of gabapentin in patients aged 65 years and older. In one double-blind study in patients with neuropathic pain, patients aged 65 years and older experienced a higher incidence of somnolence, peripheral edema, and weakness than younger patients. Other than these findings, clinical trials in this age group have not shown evidence of a different adverse event profile from that in the younger patient population.
Children
Due to the lack of data on the effects of long-term (more than 36 weeks) gabapentin therapy on learning ability, intellectual abilities and development in children, the benefits of long-term therapy should be weighed against its potential risks.
Misuse, abuse and dependence
Gabapentin may cause drug dependence, which may occur at therapeutic doses. Cases of abuse have been reported. Patients with a history of substance abuse may be at increased risk of gabapentin misuse, abuse, and dependence, and gabapentin should be used with caution in such patients. The patient's risk of misuse, abuse, or dependence should be carefully assessed before prescribing gabapentin.
Patients receiving gabapentin treatment should be monitored for symptoms of gabapentin misuse, abuse, or dependence, such as development of tolerance, dose escalation, and drug-seeking behavior.
Withdrawal symptoms
Withdrawal symptoms have been observed after discontinuation of both short-term and long-term treatment with gabapentin. Withdrawal symptoms may occur shortly after discontinuation of treatment, usually within 48 hours. The most commonly reported symptoms include anxiety, insomnia, nausea, pain, increased sweating, tremor, headache, depression, feeling abnormal, dizziness and malaise. The possible occurrence of withdrawal symptoms after discontinuation of gabapentin may indicate drug dependence (see section 4.8). The patient should be informed of this at the start of treatment. If gabapentin needs to be discontinued, it is recommended that this be done gradually over at least 1 week, regardless of the indication (see section 4.8).
Laboratory tests.
Semi-quantitative determination of total urine protein using rapid tests may result in false positive results. Therefore, it is recommended to verify such rapid test results using methods based on another analytical principle, such as the biuret test, turbidimetric method, or dye binding method, or to use these methods first.
Use during pregnancy or breastfeeding
Pregnancy.
General risks of epilepsy and the use of antiepileptic drugs (AEDs).
Women of childbearing potential, especially those planning to become pregnant, and pregnant women should be counseled about the potential risk to the fetus from both seizures and antiepileptic treatment. The need for antiepileptic treatment should be reconsidered when planning pregnancy. In women being treated for epilepsy, abrupt discontinuation of antiepileptic drugs should be avoided as this may precipitate seizures and significantly worsen the condition of the mother and child. Monotherapy should be preferred whenever possible, as multiple AED therapy may be associated with a higher risk of congenital malformations than monotherapy, depending on the AED used.
Risk associated with gabapentin therapy
Gabapentin crosses the human placenta.
There is limited evidence of an increased risk of low birth weight, preterm birth but not stillbirth, intrauterine growth retardation, low Apgar score at 5 minutes after birth, and microcephaly in infants exposed to gabapentin in utero.
Animal studies have shown reproductive toxicity.
Gabapentin can be used during the first trimester of pregnancy if clinically needed.
Neonatal withdrawal syndrome has been reported in newborns exposed to gabapentin in utero.
Concomitant use of gabapentin and opioids during pregnancy may increase the risk of neonatal withdrawal syndrome in the newborn. Newborns should be closely monitored.
Breastfeeding period.
Gabapentin is excreted in breast milk. Since the effects of the drug on breast-fed infants have not been studied, caution should be exercised when administering gabapentin to breastfeeding women. Gabapentin should be used in breastfeeding women only if the potential benefit to the mother outweighs the potential risk to the infant.
Fertility.
Animal studies have not shown any effect on fertility.
Ability to influence reaction speed when driving vehicles or other mechanisms
Gabapentin may have minor or moderate influence on the ability to drive and use machines. Gabapentin affects the central nervous system and may cause drowsiness, dizziness or other similar symptoms. These side effects, even mild or moderate, are potentially dangerous for patients when driving or operating machinery, especially at the beginning of therapy and after increasing the dose.
Method of administration and doses
The medicine is intended for oral administration.
Gabapentin can be taken with or without food. The capsule should be swallowed whole with a sufficient amount of liquid (e.g. a glass of water).
For all indications, the dose selection scheme shown in Table 1 should be used to initiate therapy. This scheme is recommended for adults and children aged 12 years and older. The dose selection scheme for children aged 6 to 12 years is described in a separate section below.
| Dosage calculation for initial dose selection. Table 1 | ||
| Day 1 | Day 2 | Day 3 |
| 300 mg once daily | 300 mg 2 times a day | 300 mg 3 times a day |
Gabapentin withdrawal
It is recommended to discontinue gabapentin gradually over a minimum of 1 week, regardless of the indication.
Epilepsy
Epilepsy usually requires long-term therapy. The dose is determined by the doctor according to individual tolerability and effectiveness.
Adults and children aged 12 and over
Effective doses for epilepsy range from 900 to 3600 mg/day. Treatment begins with titration of the drug dose as described in Table 1, or with a dose of 300 mg 3 times a day on the 1st day. Then, depending on individual tolerability and effectiveness, the dose can be increased by 300 mg/day every 2–3 days to a maximum dose of 3600 mg/day. Some patients require slower titration of gabapentin. The shortest time to reach a dose of 1800 mg/day is 1 week, 2400 mg/day is 2 weeks, and 3600 mg/day is 3 weeks.
In long-term open clinical studies, a dose of 4800 mg/day was well tolerated by patients. The daily dose should be divided into 3 doses. The maximum interval between doses should not exceed 12 hours to avoid interruptions in anticonvulsant therapy and to prevent the occurrence of seizures.
Children aged 6 to 12 years.
The starting dose of the drug should be 10–15 mg/kg/day. The effective dose should be achieved by titrating the drug over approximately 3 days. The effective dose of gabapentin for children aged 6 years and older is 25–35 mg/kg/day. A dose of 50 mg/kg/day was well tolerated by patients in long-term clinical studies. The total daily dose should be divided into equal parts (taken 3 times a day); the maximum interval between doses should not exceed 12 hours.
There is no need to monitor gabapentin serum levels. In addition, gabapentin can be used in combination with other antiepileptic drugs, as this does not change the plasma concentration of gabapentin or the serum concentration of other antiepileptic drugs.
Peripheral neuropathic pain
Adults
The efficacy and safety of gabapentin in the treatment of peripheral neuropathic pain (e.g., painful diabetic neuropathy or postherpetic neuralgia) have not been studied in long-term clinical trials lasting more than 5 months. If a patient requires longer-term (more than 5 months) treatment with gabapentin for neuropathic pain, the physician should assess the patient's clinical status and determine the need for additional therapy before continuing therapy.
Recommendations regarding the prescription of gabapentin for all indications
In patients with severe general condition or certain complicating factors, such as low body weight, post-transplant status, titration should be performed more slowly or the step dose should be reduced, or the intervals between dose increases should be prolonged.
Use in elderly patients (over 65 years of age).
Elderly patients may require individual dose adjustment due to possible decreased renal function (see Table 2). Elderly patients are more likely to develop drowsiness, peripheral edema, and weakness.
Use in patients with renal failure.
Patients with severe renal insufficiency and patients on hemodialysis require individual dose selection (see Table 2).
Doses in renal impairment Table 2
| Creatinine clearance (ml/min) | Total daily dose of gabapentin* (mg/day) |
| > 80 (creatinine clearance norms) | 900–3600 |
| 50–79 | 600–1800 |
| 30–49 | 300–900 |
| 15–29 | 150**–600 |
| <15*** | 150**–300 |
* The total daily dose should be divided into 3 doses. Reduced doses should be used in patients with renal insufficiency (creatinine clearance <79 ml/min).
** Administer at a dose of 300 mg every other day.
*** For patients with creatinine clearance <15 ml/min, the daily dose should be reduced according to creatinine clearance (e.g., patients with creatinine clearance 7.5 ml/min should receive half the daily dose of patients with creatinine clearance 15 ml/min).
Dosage for patients receiving hemodialysis.
For anuric patients on hemodialysis who have never received gabapentin before, the recommended loading dose should be 300–400 mg*, followed by 200–300 mg* of gabapentin after every 4 hours of hemodialysis. Gabapentin should not be taken on hemodialysis-free days.
The maintenance dose of gabapentin for patients on hemodialysis should be determined according to Table 2.
In addition to the maintenance dose, patients on hemodialysis are recommended to take 200–300 mg* of the drug after every 4 hours of hemodialysis.
* Use gabapentin drugs in the appropriate dosage.
Children.
Gabapentin is indicated for the treatment of children with epilepsy: as adjunctive therapy for children aged 6 years and older, and as monotherapy for children aged 12 years and older.
Overdose
Even when taking the drug in doses up to 49 g/day, the development of acute life-threatening toxic reactions was not observed.
Symptoms of overdose included dizziness, diplopia, slurred speech, drowsiness, loss of consciousness, lethargy, and mild diarrhea. All patients recovered fully with supportive care. The reduced absorption of gabapentin at high doses may limit the absorption of other drugs and reduce the toxic effects of overdose. Overdose of gabapentin, especially in combination with other CNS depressants, may result in coma.
Treatment is symptomatic. Although gabapentin can be removed by hemodialysis, this is usually not necessary. However, hemodialysis may be indicated in patients with severe renal insufficiency.
In studies in mice and rats, it was not possible to determine the lethal dose of gabapentin, despite the use of doses that reached 8000 mg/kg. Symptoms of acute toxicity in animals included: ataxia, difficulty breathing, ptosis, decreased activity or, conversely, increased excitability.
Side effects
In studies of epilepsy (adjunctive therapy or monotherapy) and neuropathic pain, the following adverse reactions were observed (listed by frequency): very common (> 1/10), common (> 1/100 - <1/10), uncommon (> 1/1,000 - <1/100) and rare (> 1/10,000 - <1/1,000). If the frequency of adverse reactions differed between studies, the highest frequency was included in the report.
Additional adverse reactions reported in post-marketing studies are included in the category "frequency unknown" (cannot be estimated from the available data).
Within each frequency grouping, undesirable effects are presented in order of decreasing severity.
Infectious and parasitic diseases
Very common: viral infection.
Common: pneumonia; respiratory infection1; urinary tract infection; ear infection, otitis media.
From the side of the hematopoietic and lymphatic systems
Common: leukopenia.
Frequency unknown: thrombocytopenia.
On the part of the immune system
Uncommon: allergic reactions (e.g. urticaria).
Frequency unknown: hypersensitivity syndrome with cutaneous and systemic manifestations, which may include symptoms such as fever, rash, hepatitis, lymphadenopathy, eosinophilia, etc. (DRESS syndrome); anaphylaxis (see section "Special warnings and precautions for use").
Metabolism and nutrition
Uncommon: hyperglycaemia (most often observed in patients with diabetes mellitus).
Rare: hypoglycemia (most often observed in patients with diabetes mellitus).
Frequency unknown: hyponatremia.
Mental disorders
Common: hostility, confusion, emotional lability, depression, anxiety, nervousness, thinking disorders.
Uncommon: agitation.
Frequency unknown: suicidal thoughts/behavior, hallucinations, drug dependence.
From the nervous system
Very common: drowsiness, dizziness, ataxia.
Common: convulsions1, hyperkinesia1, dysarthria, amnesia, tremor, insomnia, headache, sensory disturbances (paraesthesia, hypoaesthesia), coordination disorders, nystagmus, increased, decreased or absent reflexes.
Uncommon: hypokinesia, thinking disorders.
Rare: loss of consciousness.
Frequency unknown: other movement disorders (including choreoathetosis, dyskinesia, dystonia).
From the organs of vision
Common: visual disturbances, such as amblyopia or diplopia.
Hearing and balance disorders
Common: vertigo.
Frequency unknown: ringing in the ears.
From the heart
Uncommon: feeling of increased heartbeat.
From the vascular side
Common: increased blood pressure, vasodilation.
Respiratory, thoracic and mediastinal disorders
Common: dyspnea, bronchitis, pharyngitis, cough, rhinitis.
Rare: respiratory depression.
Gastrointestinal tract
Common: vomiting, nausea, dental changes, gingivitis, diarrhea, abdominal pain, dyspepsia, constipation, dry mouth or throat, flatulence.
Uncommon: dysphagia (difficulty swallowing).
Frequency not known: pancreatitis2.
Liver and biliary tract
Frequency unknown: hepatitis, jaundice.
Skin and subcutaneous tissue disorders
Common: facial swelling, purpura (most often described as bruising after injury), rash, itching, acne.
Frequency unknown: Stevens-Johnson syndrome, toxic epidermal necrolysis, eosinophilia with systemic symptoms (see section "Special warnings and precautions for use"), erythema multiforme, angioedema, alopecia.
Skeletal muscle and connective tissue disorders
Common: arthralgia, myalgia, back pain, muscle cramps.
Frequency unknown: rhabdomyolysis, myoclonic seizures.
Renal and urinary tract disorders
Frequency unknown: acute renal failure, urinary incontinence.
Reproductive system and breast disorders
Common: impotence.
Frequency unknown: breast hypertrophy, gynecomastia, sexual dysfunction (including changes in libido, ejaculation disorders, anorgasmia).
General disorders and administration site conditions
Very common: fatigue, fever.
Common: peripheral edema, gait disturbance, weakness, pain, discomfort, flu-like syndrome.
Uncommon: Generalised oedema.
Frequency not known: withdrawal reactions3, chest pain. Cases of sudden death have been described, but a clear relationship to gabapentin has not been established.
Laboratory and instrumental data
Common: decreased white blood cell count, weight gain.
Uncommon: increased liver function tests (AST, ALT) and bilirubin.
Frequency not known: increased creatine kinase4.
Injuries and poisoning
Common: accidental injuries, fractures, scratches.
Uncommon: fall.
1 Cases of respiratory tract infections, otitis media, seizures and bronchitis have only been reported in clinical studies in children. In addition, aggressive behavior and hyperkinesia were observed quite commonly in studies in children.
2 Cases of acute pancreatitis have been reported in association with gabapentin treatment. The relationship to gabapentin has not been established (see section 4.4).
3 Withdrawal symptoms have been observed after discontinuation of both short-term and long-term treatment with gabapentin. Withdrawal symptoms may occur shortly after discontinuation of treatment, usually within 48 hours. The most commonly reported symptoms include anxiety, insomnia, nausea, pain, increased sweating, tremor, headache, depression, feeling abnormal, dizziness and malaise (see section 4.4). The occurrence of withdrawal symptoms after discontinuation of gabapentin may indicate drug dependence (see section 4.8). The patient should be informed of this at the start of treatment. If gabapentin needs to be discontinued, it is recommended that this be done gradually over at least 1 week, regardless of the indication (see section 4.8).
4 Cases of myopathy with elevated creatine kinase have been reported in patients with end-stage renal disease undergoing hemodialysis.
Reporting of suspected adverse reactions.
Reporting adverse reactions after the registration of a medicinal product is important. This allows monitoring of the benefit/risk ratio when using this medicinal product. Medical and pharmaceutical professionals, as well as patients or their legal representatives, should report all cases of suspected adverse reactions and lack of efficacy of the medicinal product via the Automated Information System for Pharmacovigilance at the link: https://aisf.dec.gov.ua.
Expiration date
2 years.
Storage conditions
Store at a temperature not exceeding 25 ºС.
Keep out of reach of children.
Packaging
10 capsules in a blister. 1, 3 or 10 blisters in a cardboard box.
Vacation category
According to the recipe.
Producer
KUSUM HEALTHCARE PVT LTD/KUSUM HEALTHCARE PVT LTD.
Location of the manufacturer and address of its place of business.
Plot No. M-3, Indore Special Economic Zone, Phase-II, Pithampur, Distt. Dhar, Madhya Pradesh, Pin 454774, India.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.