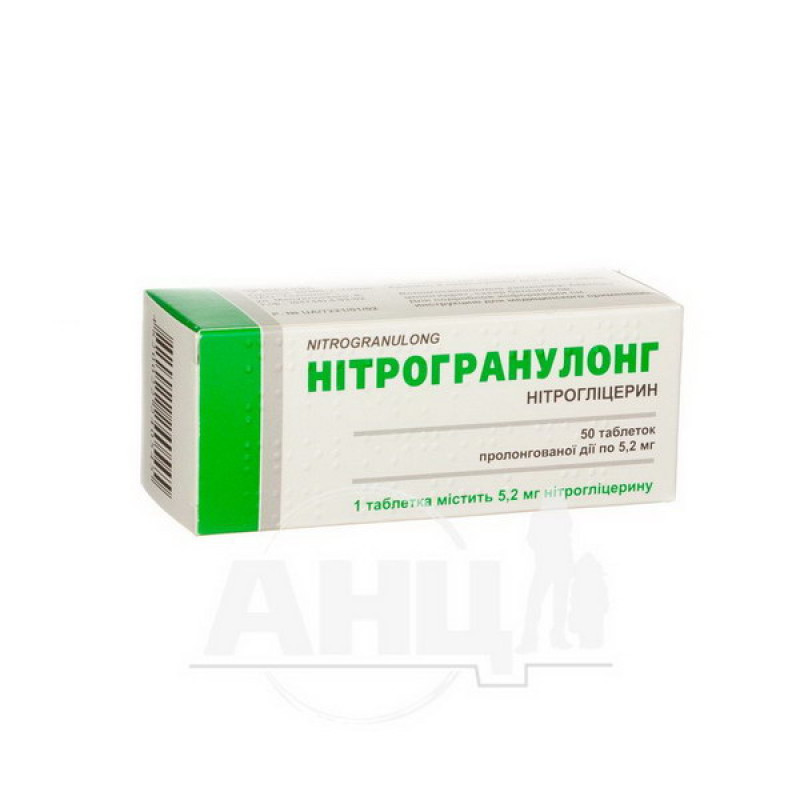
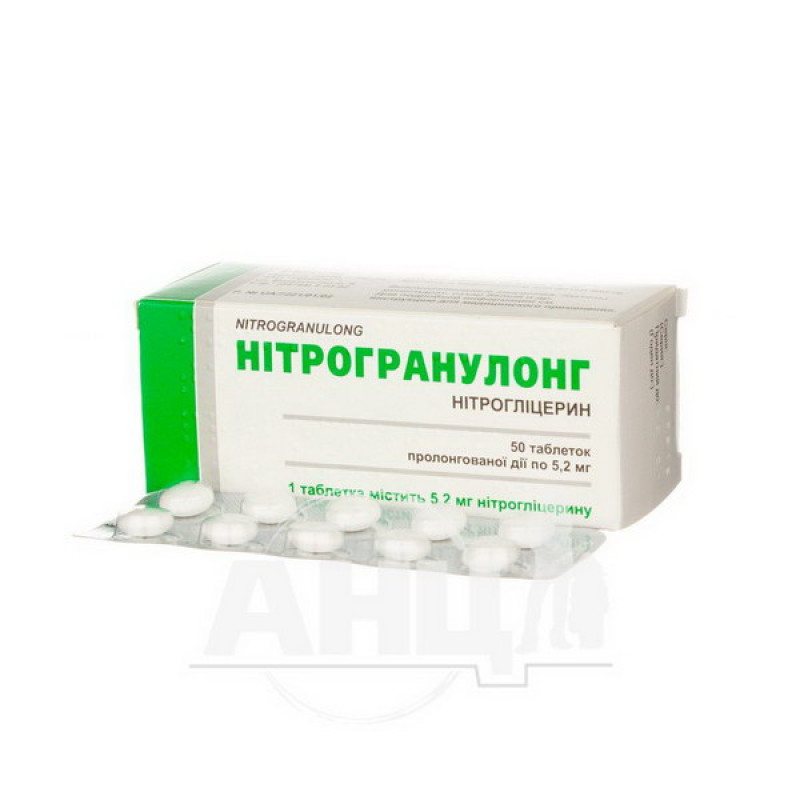
Nitrogranulong prolonged-release tablets 5.2 mg blister No. 50
Group: Drugs affecting the cardiovascular system
Active ingredient: Nitroglycerin 2% on lactose
General characteristics
composition:
active ingredient: nitroglycerin;
1 tablet contains 2.9 mg or 5.2 mg of nitroglycerin;
excipients: lactose, potato starch, talc, magnesium stearate, povidone, white sugar, corn starch, colloidal anhydrous silicon dioxide, titanium dioxide (E 171), gelatin, polyethylene glycol 6000 (macrogol 6000).
Dosage form.
Extended-release tablets.Pharmacotherapeutic group.
Vasodilators used in cardiology. Glyceryl trinitrate. ATC code C01 D A02.Clinical characteristics.
Indication.
Prevention of angina attacks in patients with coronary heart disease (CHD), including in the post-infarction period.
Contraindication.
Hypersensitivity, arterial hypotension (BP below 90/60 mm Hg), acute myocardial infarction with low right ventricular filling pressure, toxic pulmonary edema, hemorrhagic stroke, intracranial hypertension, angle-closure glaucoma, acute anemia, shock, collapse, hypertrophic obstructive cardiomyopathy, simultaneous use with sildenafil and other phosphodiesterase inhibitors, bradycardia (less than 50 beats / min), cerebral ischemia, cardiac tamponade.
Method of administration and doses.
Dosage depends on the individual patient and the severity of the disease. To prevent the development of tolerance, the dosage regimen should include a 10-12 hour nitrate-free interval.
In mild cases, Nitrogranulong is taken 1-2 tablets of 2.9 mg twice a day, in the morning and after lunch. In more severe cases, 1-2 tablets of 5.2 mg twice a day, in the morning and after lunch. The absence of an evening dose ensures a 12-hour interval. If necessary, the drug may be prescribed 3 times a day, but with a 10-12-hour nitrate-free interval.
If the patient's attacks occur mainly at night, Nitrogranulong should be taken after lunch and in the evening.
The tablets should be taken on an empty stomach, swallowed whole, without breaking or chewing, with a small amount of liquid.
The maximum daily dose should not exceed 30 mg.
Adverse reactions.
In the initial stages of drug use (1-2 days), depending on the dose and most often due to the vasodilator effect, the following adverse reactions may be observed.
From the side of the central nervous system: headache, dizziness, fainting, anxiety, psychotic reactions, inhibition, disorientation.
Cardiovascular system: orthostatic reactions, decreased blood pressure, tachycardia, paradoxical bradycardia (with acute hypotension or syncope), facial flushing, cyanosis, pallor, methemoglobinemia.
On the part of the digestive system: nausea, vomiting, dry mouth, abdominal pain, diarrhea.
On the part of the immune system: allergic reactions, including skin rashes, itching, redness of the skin; anaphylactic shock.
Others: general weakness, feeling of heat, visual impairment, exacerbation of glaucoma, hypothermia.
With a sudden drop in blood pressure, an increase in angina symptoms may occur (paradoxical "nitrate" reactions).
Overdose.
Symptoms: headache, hypotension, palpitations, visual disturbances, erythema, increased sweating, nausea, vomiting, cyanosis, bradycardia, convulsions and coma, in acute cases - methemoglobinemia, severe dizziness, fainting, shortness of breath, severe weakness, drowsiness, increased body temperature, feeling of heat, chills. When using high doses (more than 20 mcg/kg) - collapse, shortness of breath and tachypnea.
Treatment: gastric lavage, administration of activated charcoal, laxatives, restoration of normal respiratory function and blood pressure (lying position on a low pillow, fluid infusions). To eliminate methemoglobinemia, the drug of choice is methylene blue.
Use during pregnancy or breastfeeding.
The use of nitroglycerin during pregnancy or breastfeeding is contraindicated.
Children.
The drug is not used in pediatrics.
Application features.
Nitrogranulong is not prescribed for the treatment of acute attacks of angina pectoris.
It should be borne in mind that uncontrolled use of the drug may lead to the development of tolerance to nitrates, which is expressed in a decrease in the duration and severity of the drug's effect with regular use or an increase in its dose to achieve the same effect. To prevent refractoriness, intermittent use of the drug is necessary throughout the day.
It is necessary to take the drug with caution, taking into account the risk and benefit, in the following cases: uncontrolled hypovolemia, heart failure with normal or low pulmonary artery pressure, hyperthyroidism, impaired cerebral circulation, severe renal and/or hepatic insufficiency (risk of developing methemoglobinemia). Use with caution in aortic stenosis. It should be used with caution in patients with severe cerebral atherosclerosis, elderly patients. Do not drink alcohol during treatment. During treatment, visiting a bathhouse, sauna, or taking a hot shower is contraindicated. The tablet should not be chewed, since an excessive amount of the active substance may enter the systemic bloodstream through the oral mucosa.
The drug contains lactose, therefore patients with rare hereditary forms of galactose intolerance, lactase deficiency or glucose-galactose malabsorption syndrome should not use the drug.
The ability to influence the reaction speed when driving or working with other mechanisms.
While taking the drug, you should not drive vehicles, operate mechanical devices, or perform work that requires increased attention and rapid psychomotor reactions.
Interaction with other drugs and other types of interactions.
When used simultaneously with other antihypertensive agents, phosphodiesterase inhibitors, procainamide, ACE inhibitors, calcium channel blockers, beta-blockers, diuretics, tricyclic antidepressants, MAO inhibitors, ethanol and ethanol-containing drugs, the hypotensive effect of nitroglycerin is enhanced; with beta-blockers, calcium channel blockers - the antianginal effect is enhanced; with dihydroergotamine - an increase in its plasma concentration is possible. The use of nitroglycerin on the background of quinidine or novocainamide can cause orthostatic collapse.
Simultaneous intake of ergot alkaloids may cause angina. With simultaneous use of alcohol, side effects of the drug may be more severe and more frequent.
Atropine and other drugs that have M-cholinolytic effects can reduce the effect of nitroglycerin due to a decrease in secretion and bioavailability of the drug. When used simultaneously with heparin, the anticoagulant effect of the latter may decrease (after discontinuation of the drug, a significant decrease in blood clotting is possible, which may require a decrease in the dose of heparin). Phenobarbital activates the metabolism of nitrates in the liver. Alpha-adrenomimetics, histamine, pituitrin, corticosteroids, CNS stimulants, bee venom, snake venom, sunlight reduce the antianginal effect of nitroglycerin. Salicylates increase the level of nitroglycerin in the blood, barbiturates accelerate its metabolism. Donors of sulfhydryl groups (captopril, acetylcysteine, unitiol) restore reduced sensitivity to nitroglycerin.
Pharmacological properties.
Pharmacodynamics.
Antianginal drug of prolonged action. Nitroglycerin, belonging to the group of organic nitrates, is an active vasodilator that acts on both arterial and venous vessels. The mechanism of the antianginal effect of nitroglycerin drugs of prolonged action is associated with its peripheral vasodilating effect. Along with reducing the resistance of coronary vessels, nitroglycerin dilates mainly postcapillary venous vessels, leading to a decrease in venous return to the heart; in higher doses, it also dilates precapillary arterioles, which regulate vascular resistance, as a result of which the work of the heart and its need for oxygen are reduced. The drug promotes the redistribution of coronary blood flow in the area with reduced blood circulation. Increases tolerance to physical exertion in patients with angina.
Pharmacokinetics.
After taking the drug orally, nitroglycerin is gradually absorbed in the small intestine; the effect occurs after 30-60 minutes and lasts 4-6 hours. The drug is largely destroyed in the liver with the participation of nitrate reductase (the "first-pass" effect), and then biotransformed into nitric oxide (NO) in smooth muscle cells. Bioavailability is no more than 10% compared to nitroglycerin used sublingually. Metabolites are di- and mononitrates (only isosorbide-5-mononitrate is active), the final one is glycerol. The half-life of metabolites is 4 hours. In plasma, it binds to proteins (60%). Metabolites are extracted mainly by the kidneys.
Pharmaceutical characteristics.
Main physicochemical properties: round, film-coated tablets, white or almost white in color, with convex upper and lower surfaces. When broken, when viewed under a magnifying glass, a core surrounded by one continuous layer is visible.
Expiration date.
3 years.Storage conditions.
Store in original packaging at a temperature not exceeding 25 0 C. Keep out of the reach of children.
Packaging.
10 tablets in blisters;
10 tablets in a blister, 5 blisters in a cardboard pack;
50 tablets in a container, 1 container in a cardboard pack.
Release category: By prescription.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.