Normolact syrup bottle 200 ml

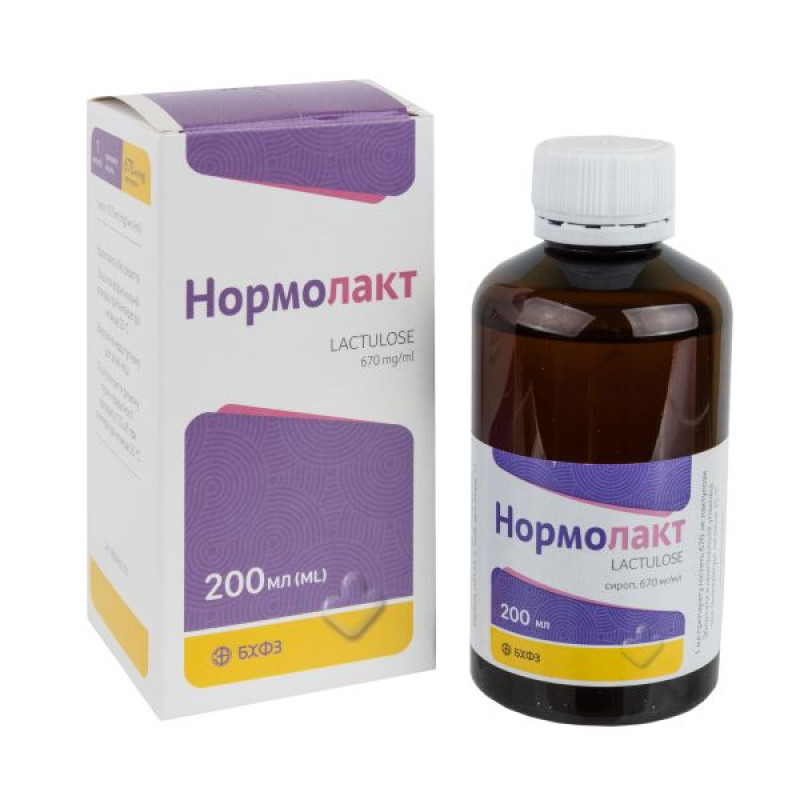
Instructions Normolact syrup bottle 200 ml
Composition
active ingredient: lactulose;
1 ml of syrup contains 670 mg of lactulose.
Dosage form
Syrup.
Main physicochemical properties: transparent, viscous liquid, colorless or slightly brownish-yellow in color.
Pharmacotherapeutic group
Osmotic laxatives. Lactulose.
ATX code A06A D11.
Pharmacological properties
Pharmacodynamics
Lactulose is a synthetic derivative of lactose. It does not break down in the stomach and small intestine due to the lack of appropriate enzymes and is practically not absorbed. In the large intestine, lactulose is broken down under the action of microflora into low-molecular organic acids, as a result of which the pH decreases and osmotic changes occur that stimulate intestinal peristalsis. The volume of feces also increases, which contributes to the normalization of the defecation process. Lactulose as a prebiotic enhances the growth of bifido- and lactobacteria, due to which the growth of pathogenic intestinal microflora is suppressed (in particular, pathogens such as clostridia and E. coli).
In hepatic encephalopathy or hepatic (pre)coma, the therapeutic effect of lactulose is associated with inhibition of the growth of proteolytic bacteria by increasing the number of acidophilic bacteria (e.g. lactobacilli), transformation of ammonia into an ionized form due to acidification of intestinal contents, laxative effect due to low pH and osmotic effect, and change in nitrogen metabolism in bacteria by stimulating the utilization of ammonia by bacteria for protein synthesis.
Pharmacokinetics
The drug is almost not absorbed in the intestine and at a dose of 40-75 ml is completely metabolized by bacterial flora. When using higher doses, part of lactulose may be excreted unchanged.
Indication
Constipation: regulation of the physiological rhythm of the intestine;
conditions requiring relief of defecation (hemorrhoids, after operations on the colon and anorectal area);
Portosystemic hepatic encephalopathy (PSE): treatment and prevention of hepatic precoma and coma.
Contraindication
Hypersensitivity to the active substance or to other components of the drug;
galactosemia;
acute pain in the abdominal area of unknown origin;
nausea, vomiting;
gastrointestinal obstruction/intestinal stenosis;
perforation of the digestive tract or risk of perforation of the digestive tract (for example, acute inflammatory bowel diseases such as ulcerative colitis, Crohn's disease);
rectal bleeding;
severe dehydration.
Interaction with other medicinal products and other types of interactions
If you are taking any other medications, be sure to tell your doctor.
When taking Normolact with enteric-coated drugs of pH-dependent release, it is worth remembering that lactulose lowers the pH of the intestine.
When used simultaneously with broad-spectrum antibiotics or antacids, the therapeutic efficacy of lactulose may be reduced.
Lactulose may increase potassium loss induced by other drugs (e.g., thiazides, corticosteroids, and amphotericin B).
Concomitant use with cardiac glycosides may enhance the effects of glycosides due to potassium deficiency.
A synergistic effect with neomycin is possible.
It is not recommended to take Normolact within 2 hours of taking other medications.
Application features
Before starting treatment with Normolact, you should consult a doctor, as the doctor must determine the dosage of the drug and the duration of treatment.
A doctor's consultation is recommended if:
– before starting treatment, there are painful symptoms in the abdominal area of unknown origin;
– the therapeutic effect within several days of treatment is insufficient.
Given the amount of sugar in the product, the dose typically used to treat constipation does not pose a problem for diabetic patients. However, much higher doses are typically prescribed for the treatment of hepatic (pre)coma, so the sugar content of the product should be taken into account when treating diabetic patients.
100 ml of syrup contains 1.4 bread units.
In case of gastrocardial syndrome, the dose should be increased gradually to avoid flatulence.
Long-term use of the drug (more than 6 months) without dose adjustment or incorrect use may lead to diarrhea and electrolyte imbalance. In serious cases, dehydration or hypokalemia may occur. Hypokalemia may cause cardiac or neuromuscular dysfunction, especially in case of concomitant treatment with cardiac glycosides, diuretics or corticosteroids. Plasma electrolyte levels should be regularly monitored, especially in elderly and debilitated patients.
The drug contains lactose, galactose and a small amount of fructose, therefore patients with rare hereditary problems of galactose or fructose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this drug.
If the signs of the disease do not disappear or, on the contrary, the state of health worsens, or undesirable phenomena appear, it is necessary to stop taking the drug and consult a doctor regarding further use.
Use during pregnancy or breastfeeding
Pregnancy.
During pregnancy, no effects on the fetus are expected, since the systemic effect of lactulose on the pregnant woman is insignificant. If necessary, Normolact can be used during pregnancy.
Breastfeeding period.
No effects on the newborn/infant are expected during breastfeeding, as the systemic exposure of lactulose to the mother is negligible. Normolact can be used during breastfeeding.
Fertility
No effects are expected, as the systemic exposure to lactulose is negligible.
Ability to influence reaction speed when driving vehicles or other mechanisms
Taking Normolact has no or negligible effect on the ability to drive or use machines.
Method of administration and doses
Take internally, both diluted and undiluted.
The dose should be selected based on the clinical effect.
The dosage regimen should be selected depending on the individual needs of the patient.
It is undesirable to exceed the recommended doses.
Dosage for constipation or conditions requiring easier bowel movements
| Age group | Initial daily dose | Maintenance daily dose |
| ml | ml | |
| Children under 1 year old | 5 | 5 |
| Children aged 1 to 6 years | 5-10 | 510 |
| Children 7-14 years old | 15 | 10-15 |
| Adults and children aged 14 and over | 15-45 | 15-30 |
*For children under 1 year of age, the dose of the drug is measured according to the scale indicated on the measuring spoon that is included.
It is better to take a single dose in one go (without keeping it in the mouth for a long time) always at the same time of day, for example in the morning during breakfast. It is possible to mix it with fruit and vegetable juices or food mixtures. The effect can be observed after 1-2 days, which is due to the action of lactulose.
During laxative therapy, it is recommended to consume sufficient amounts of fluids (1.5-2 liters, which corresponds to 6-8 glasses) per day.
In hepatic encephalopathy, hepatic coma and precoma, the drug is prescribed in an initial dose for adults of 30-45 ml 3-4 times a day.
The maintenance dose should be set depending on the individual response (2-3 soft stools per day should be achieved).
The safety and efficacy of the drug in children (0-18 years) with portosystemic encephalopathy have not been established. Data are not available.
Elderly patients and patients with renal or hepatic insufficiency.
Since the systemic exposure to lactulose is negligible, there are no specific dosage recommendations for these patient groups.
Children
Laxatives should only be used in children in exceptional cases and under the supervision of a doctor.
There are no data on the use of the drug in children with hepatic encephalopathy.
It should be borne in mind that the bowel emptying reflex may be impaired during treatment.
Overdose
Symptoms: Laxative abuse (frequent or prolonged use in excessive doses) can lead to abdominal pain and persistent diarrhea with subsequent loss of water, mineral salts (especially potassium) and other essential nutrients. In serious cases, dehydration or hypokalemia may occur. Hypokalemia may cause cardiac or neuromuscular dysfunction, especially in the case of concomitant treatment with cardiac glycosides, diuretics or corticosteroids.
Treatment: there is no specific antidote, it is recommended to reduce the dose or stop taking the drug. Excessive fluid loss due to vomiting and diarrhea may require correction of electrolyte balance. After stopping Normolact, these symptoms disappear.
Adverse reactions
During the first days of treatment, flatulence may occur, which usually disappears after a few days. When using the drug in doses exceeding the recommended ones, abdominal pain and diarrhea may occur. In this case, the dose should be reduced. When using high therapeutic doses for a long time (usually only in hepatic encephalopathy), electrolyte imbalance may occur due to diarrhea.
The following adverse reactions occurred with the following frequencies in patients treated with lactulose in placebo-controlled clinical trials: very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1,000 to <1/100), rare (≥1/10,000 to <1/1,000), very rare (<1/10,000).
Gastrointestinal tract: very often - diarrhea; often - flatulence, abdominal pain or colic, nausea and vomiting.
Skin and subcutaneous tissue disorders: not known - rash, pruritus, urticaria.
Laboratory abnormalities: uncommon - electrolyte imbalance due to diarrhea (usually only in patients with portosystemic encephalopathy).
Other: headache, dizziness, fatigue, weakness, myalgia and arrhythmia.
Expiration date
2 years.
Do not use the drug after the expiration date indicated on the package.
After opening the bottle, the shelf life of the drug is 120 days at a temperature not exceeding 25°C.
Storage conditions
Store in the original packaging at a temperature not exceeding 25 ºС.
Keep out of reach of children.
Packaging
100 ml in a polymer jar, 1 jar with a dosing spoon in a pack;
100 ml in a polymer bottle, 1 bottle together with a dosing spoon in a pack;
200 ml in a polymer bottle, 1 bottle together with a dosing spoon in a pack;
240 ml in a polymer bottle, 1 bottle together with a dosing spoon in a pack;
500 ml in a polymer bottle, 1 bottle together with a dosing spoon in a pack.
Vacation category
Without a prescription.
Producer
Public Joint Stock Company "Research and Production Center "Borshchagov Chemical and Pharmaceutical Plant".
Location of the manufacturer and address of its place of business
Ukraine, 03134, Kyiv, Myru St., 17.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.