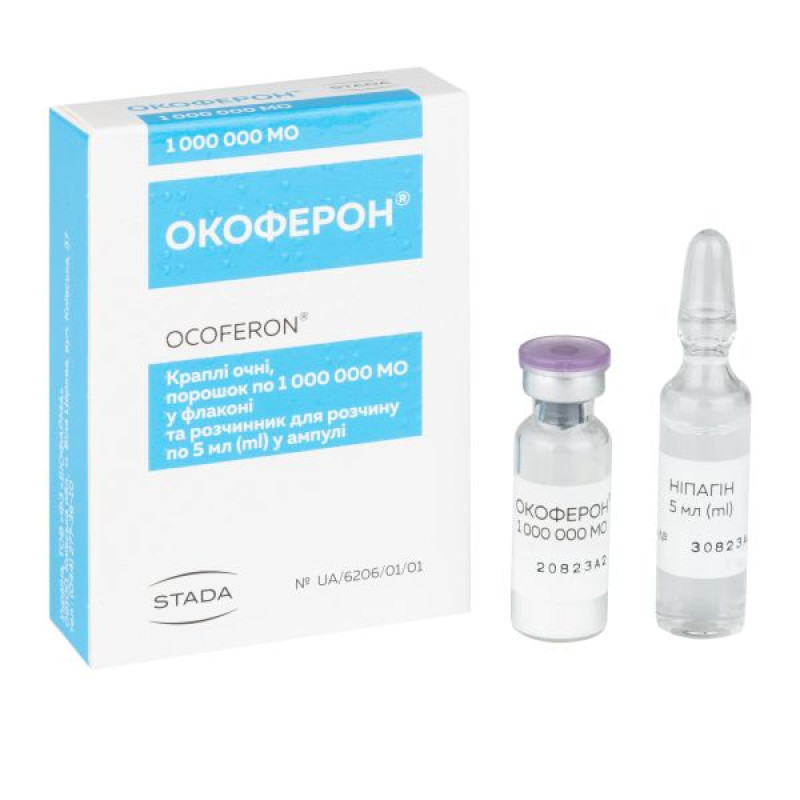
Okoferon eye drops powder 1000000 IU bottle with solvent in a 5 ml bottle
Instructions Okoferon eye drops powder 1000000 IU bottle with solvent in a 5 ml bottle
Composition
active ingredient: 1 bottle of the drug contains 1,000,000 IU of recombinant human interferon alpha-2b;
excipients: sodium hydrogen phosphate dodecahydrate, sodium dihydrogen phosphate dihydrate, dextran-70, sodium chloride;
solvent for Okoferon®: 1 ampoule of solvent (5 ml) contains: methyl parahydroxybenzoate (nipagin) (E 218) – 5 mg, water for injection.
Dosage form
Eye drops, powder.
Main physicochemical properties: lyophilized porous mass of white color.
Pharmacotherapeutic group
Means used in ophthalmology. ATX code S01X A.
Pharmacological properties
Pharmacodynamics.
The drug exhibits immunomodulatory and antiviral activity.
Interferon alfa-2b interacts with the corresponding receptors on the cell surface. Thus, processes are activated that prevent viral replication inside the cell and slow down cell proliferation. Immunomodulatory effect: interferon alfa-2b stimulates the phagocytic activity of macrophages, as well as the cytotoxic activity of T-cells and natural killer cells.
Pharmacokinetics.
Didn't study.
Indication
Viral eye infections (herpetic infection).
Contraindication
Hypersensitivity to the components of the drug.
Interaction with other medicinal products and other types of interactions
In order to avoid possible physicochemical interaction of Okoferon® with other ophthalmic agents, it is advisable to apply it 30 minutes before or 30 minutes after instillation of other medications into the eye.
Application features
Use methyl parahydroxybenzoate as a solvent, which may cause allergic reactions, including delayed ones, and in exceptional cases, bronchospasm.
Treatment should be started at the first signs of viral eye infection. The drug does not have a systemic effect. In viral infections, Okoferon® should be used in combination with recombinant human interferon alpha-2b preparations for systemic use (injections, suppositories).
Use during pregnancy or breastfeeding
There is no experience of use during pregnancy or breastfeeding.
The ability to influence the reaction speed when driving or working with other mechanisms
Since in rare cases local reactions are possible after taking the drug, you should not drive or operate other mechanisms immediately after use.
Method of administration and doses
Before use, Okoferon® should be dissolved in 5 ml of solvent (0.1% nipagin solution). 1 ml of the finished solution contains 200,000 IU of recombinant human interferon alpha-2b.
The drug is instilled 2 drops into the conjunctival sac of the affected eye every 2 hours, but not less than 6 times a day. As the symptoms of the disease decrease, the volume of instillations can be reduced to 1 drop. The course of treatment is 7-10 days.
Children
There is no experience in children.
Overdose
Not observed.
Side effects
In rare cases, local reactions are possible, including local allergic reactions, swelling and hyperemia of the eyelids, itching, facial hyperemia, which disappear after discontinuation of the drug.
Expiration date
2 years.
Storage conditions
Store in the original packaging to protect from light at a temperature of 2 to 8°C. Keep out of the reach of children. Shelf life after opening the bottle is 28 days.
Packaging
1,000,000 IU of the drug in a glass vial. 5 ml of solvent in a glass ampoule. A polyethylene dropper cap and a Pasteur pipette are included.
Vacation category
Without a prescription.
Producer
LLC "FZ "BIOPHARMA", Ukraine.
Address
Ukraine, 09100, Kyiv region, Bila Tserkva, Kyivska st., 37.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.