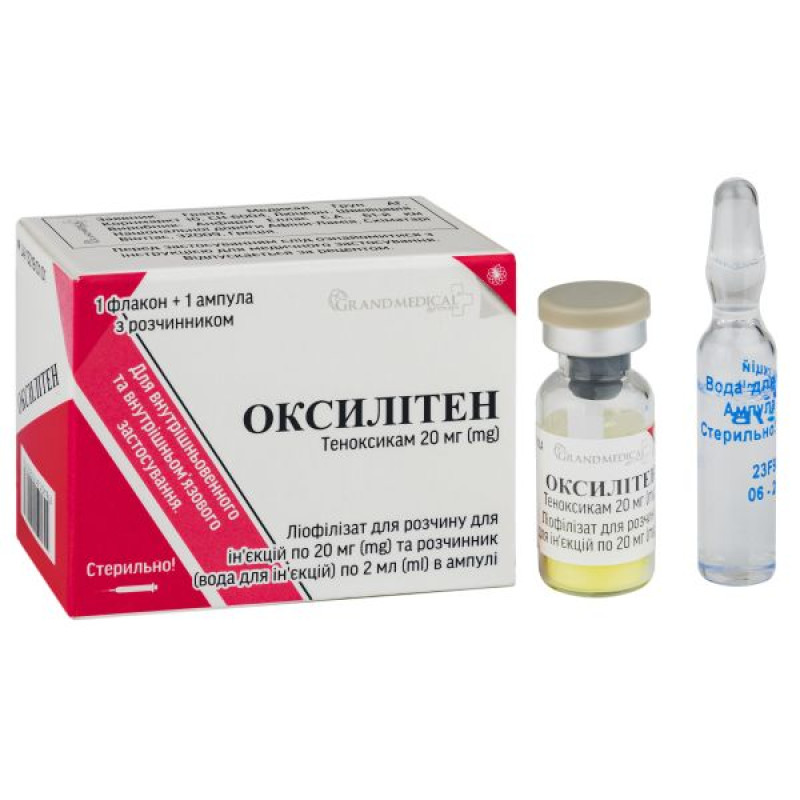
Oxyliten lyophilized powder for solution for injection 20 mg vial with solvent in ampoules 2 ml No. 1
Instructions Oxylitene lyophilized powder for solution for injection 20 mg vial with solvent in ampoules 2 ml No. 1
Composition
active ingredient: tenoxicam;
1 vial contains tenoxicam 20 mg;
excipients: mannitol (E 421), sodium hydroxide, trometamol, sodium metabisulfite, disodium edetate.
1 ampoule with solvent contains 2 ml of water for injections.
Dosage form
Lyophilisate for solution for injection.
Main physicochemical properties: yellow or yellowish-green compacted powder.
Pharmacotherapeutic group
Nonsteroidal anti-inflammatory and antirheumatic drugs. Oxicam. Tenoxicam.
ATX code M01A C02.
Pharmacological properties
Pharmacodynamics.
Tenoxicam is a nonsteroidal anti-inflammatory drug. It has a pronounced analgesic, anti-inflammatory, and some antipyretic effect.
As with other nonsteroidal anti-inflammatory drugs (NSAIDs), the exact mechanism of action is unknown, although it is likely to be multifactorial, including inhibition of prostaglandin biosynthesis and reduction of leukocyte recruitment to the site of inflammation.
Pharmacokinetics.
Tenoxicam in the form of a lyophilisate is a long-acting drug, and once-daily dosing is effective.
Tenoxicam penetrates well into synovial fluid, where its concentration is approximately half of its concentration in blood plasma.
After intravenous administration of tenoxicam at a dose of 20 mg, its plasma level decreases rapidly during the first 2 hours, which is associated with the distribution process. After intramuscular injection, a level of 90% or more of the maximum concentration is reached after at least 15 minutes.
At the recommended dosage of 20 mg per day, steady-state plasma concentrations are reached within 10–15 days. Cumulation is not expected.
The drug binds strongly to blood plasma proteins.
Tenoxicam is almost completely metabolized in the body. Approximately 2/3 of the administered dose is excreted in the urine as the pharmacologically inactive metabolite 5-hydroxypyridyl, the remainder in the bile, mainly as glucuronide conjugates of hydroxymetabolites.
No changes in tenoxicam pharmacokinetics were found depending on the patient's age, although individual differences are generally greater in elderly patients.
Indication
Relief of pain and inflammation in osteoarthritis and rheumatoid arthritis.
Short-term treatment of acute musculoskeletal conditions, including sprains, dislocations, and other soft tissue injuries.
For these indications, the drug should be administered intravenously or intramuscularly if it is not possible to use tenoxicam in tablet form.
Contraindication
Hypersensitivity to tenoxicam or to any of the excipients of the drug. History of hypersensitivity symptoms (including asthma symptoms, rhinitis, angioedema or urticaria) to other NSAIDs, including ibuprofen and acetylsalicylic acid, due to possible cross-sensitivity to tenoxicam.
Recurrent active or history of gastrointestinal ulcer/bleeding (2 or more severe episodes of ulcer or bleeding), ulcerative colitis, Crohn's disease, severe gastritis, or gastrointestinal bleeding or perforation associated with previous NSAID use.
Severe heart, liver, kidney failure.
The last trimester of pregnancy.
Interaction with other medicinal products and other types of interactions
Anticoagulants: No clinically significant interaction was observed between tenoxicam in the form of a lyophilisate and low molecular weight heparin in healthy volunteers. Tenoxicam is highly bound to serum albumin and, like other NSAIDs, may enhance the anticoagulant effect of warfarin and other anticoagulants (see section "Special warnings and precautions for use"). The effect of anticoagulants and oral hypoglycemic agents should be monitored particularly carefully during the initial stages of treatment with tenoxicam.
Antiplatelet agents and selective serotonin reuptake inhibitors (SSRIs): increased risk of gastrointestinal bleeding (see section "Special warnings and precautions for use").
Antihypertensives: Tenoxicam and other NSAIDs may weaken the effect of antihypertensives.
Cardiac glycosides: NSAIDs may exacerbate heart failure, reduce glomerular filtration rate, and increase plasma levels of cardiac glycosides when used concomitantly.
Cyclosporine: As with all NSAIDs, caution is recommended when used concomitantly with cyclosporine as the risk of nephrotoxicity is increased.
Cimetidine: no interaction was found when used simultaneously with cimetidine.
Diuretics: reduction of diuretic effect. NSAIDs can retain potassium, sodium and fluid and affect the natriuretic effect of diuretics, increasing the risk of nephrotoxicity of NSAIDs. These properties should be borne in mind when treating patients with arterial hypertension or heart failure, since tenoxicam may worsen the course of these diseases.
Lithium: Decreased lithium elimination has been reported with NSAIDs. If tenoxicam is prescribed to a patient receiving lithium therapy, it is recommended that lithium levels be monitored more frequently and that the patient be advised to maintain adequate fluid intake and to be aware of the symptoms of lithium toxicity.
Methotrexate: Caution is recommended when used concomitantly with methotrexate due to the possibility of methotrexate intoxication, as NSAIDs have been reported to reduce its elimination.
Mifepristone: NSAIDs should not be used for 8–12 days after taking mifepristone, as such agents may reduce the effect of mifepristone.
NSAIDs, selective cyclooxygenase-2 (COX-2) inhibitors, salicylates: the simultaneous use of 2 or more NSAIDs (including acetylsalicylic acid) should be avoided due to the possible increased risk of adverse reactions (see section "Special instructions"). Salicylates may displace tenoxicam from protein binding, increasing its clearance and distribution. Concomitant treatment with salicylates or other NSAIDs should be avoided due to the risk of increased adverse reactions (especially from the gastrointestinal tract).
Penicillinamine, parenteral gold preparations: No clinically significant interaction was observed in a small number of patients receiving these agents concomitantly.
Quinolone antibiotics: Preclinical data suggest that NSAIDs increase the risk of quinolone-induced seizures. Concomitant use of these agents may increase the risk of seizures in patients.
Tacrolimus: There may be an increased risk of nephrotoxicity when NSAIDs are used with tacrolimus.
Zidovudine: There is a possible increased risk of hematological toxicity when NSAIDs are used with zidovudine. There is evidence of an increased risk of hemarthrosis and hematomas in HIV-infected patients with hemophilia when zidovudine and ibuprofen are used concomitantly.
Application features
Concomitant use of the drug with NSAIDs, including selective COX-2 inhibitors, should be avoided (see section "Interaction with other medicinal products and other types of interactions").
Adverse reactions to tenoxicam can be minimised by using the lowest effective dose for the shortest duration necessary to control symptoms (see section “Dosage and Administration” and information on gastrointestinal and cardiovascular risks below).
Cardiovascular and cerebrovascular effects
Patients with hypertension and/or a history of mild or moderate heart failure should be carefully monitored during use of the drug, as edema and fluid retention have been reported with NSAID treatment.
Clinical trials and epidemiological data suggest that the use of some NSAIDs, especially at high doses and over long periods, may slightly increase the risk of thrombotic events (e.g. myocardial infarction or stroke). At present, there is insufficient data to exclude such a risk for tenoxicam.
Patients with uncontrolled hypertension, congestive heart failure, established ischemic heart disease, peripheral arterial disease and/or cerebrovascular disease should only be treated after careful assessment. A similar assessment should be made before initiating long-term treatment in patients with risk factors for cardiovascular disease (such as hypertension, hyperlipidemia, diabetes mellitus, smoking).
Cardiovascular, renal and hepatic disorders
The use of NSAIDs may cause a dose-dependent decrease in prostaglandin formation and rapid renal failure. The risk of such reactions is higher in patients taking diuretics and in the elderly. In such patients, renal function should be monitored (see section "Contraindications").
The use of NSAIDs in rare cases can cause interstitial nephritis, glomerulonephritis, papillary necrosis or nephrotic syndrome due to inhibition of renal prostaglandin synthesis, which maintains renal perfusion in patients with reduced renal blood flow and total blood volume. In such patients, the use of NSAIDs can cause pronounced renal decompensation, which after discontinuation of their use returns to the state observed before the start of therapy. The greatest risk of such complications exists in patients with existing kidney diseases (including diabetes with impaired renal function), nephrotic syndrome, reduced total blood volume, impaired liver and heart function, in patients who are simultaneously using diuretics or potentially nephrotoxic drugs. During the use of the drug in such patients, kidney, liver and heart function should be constantly monitored. Patients with impaired kidney, liver and heart function should use the drug in the lowest possible dose. NSAIDs should be used with caution in patients with a history of heart failure or hypertension, as edema has been reported with ibuprofen.
Dermatological effects
The use of NSAIDs can rarely cause severe skin reactions, sometimes fatal, including exfoliative dermatitis, Stevens-Johnson syndrome and toxic epidermal necrolysis (see section "Adverse reactions"). The risk of developing such reactions is greatest at the beginning of treatment: in most cases the first manifestations were noted during the first month of therapy. At the first signs of skin rash, lesions of the mucous membranes or other signs of hypersensitivity, the drug should be discontinued immediately.
Use in elderly patients
When NSAIDs are used in elderly patients, the frequency of adverse reactions, especially gastrointestinal bleeding and perforation, including fatal ones, is increased (see section "Method of administration and dosage"). When using the drug in elderly patients, special caution should be exercised and their condition should be regularly monitored to identify possible interactions with concomitant medications, and the function of the kidneys, liver and cardiovascular system, which may be affected by NSAIDs, should be regularly checked.
Impact on female fertility
The drug may affect female fertility and is therefore not recommended for use in women attempting to conceive. Discontinuation of the drug should be considered in women who have difficulty conceiving or are undergoing investigation of infertility.
Gastrointestinal bleeding, ulcers and perforations
NSAIDs should be used with caution in patients with a history of gastrointestinal disease.
Gastrointestinal bleeding, ulceration and perforation, including fatal cases, have been reported with all NSAIDs, and can occur at any time during treatment, with or without warning symptoms, and with or without a history of gastrointestinal disease.
The risk of such events increases with increasing NSAID dose in patients with a history of gastrointestinal ulcer, especially complicated by bleeding or perforation (see section "Contraindications"), as well as in elderly patients. In such patients, treatment should be started with the lowest possible dose. For these patients, as well as for those taking concomitant low-dose acetylsalicylic acid or other agents that increase the risk of gastrointestinal complications, combination therapy with drugs such as misoprostol or proton pump inhibitors should be considered.
Patients, especially the elderly, with a history of toxic gastrointestinal lesions should be informed of any unusual symptoms occurring from the gastrointestinal tract, especially bleeding. This is especially important at the beginning of treatment.
The drug should be used with caution in patients who are concomitantly receiving drugs that increase the risk of ulceration or bleeding, such as oral corticosteroids, anticoagulants (such as warfarin), selective serotonin reuptake inhibitors or antiplatelet agents (such as acetylsalicylic acid) (see section "Interaction with other medicinal products and other types of interactions").
Patients with symptoms of gastrointestinal disease who are treated with tenoxicam should be closely monitored. If gastrointestinal bleeding or ulceration occurs, the drug should be discontinued immediately.
NSAIDs should be used with caution in patients with a history of gastrointestinal diseases (ulcerative colitis, Crohn's disease), as tenoxicam may exacerbate their manifestations (see section "Adverse reactions").
Hematological effects
Tenoxicam reduces platelet aggregation and may increase bleeding time, which should be taken into account when major surgical interventions are planned (e.g. joint replacement) and when it is necessary to determine bleeding time.
Ophthalmological effects
Visual disturbances have been reported with NSAIDs. If such disturbances develop during use of the drug, an ophthalmological examination should be performed.
The drug should be used with caution in patients with bronchial asthma or a history of bronchial asthma, since taking NSAIDs may provoke the development of bronchospasm in such patients.
Use in patients with systemic lupus erythematosus (SLE) and mixed connective tissue diseases
When NSAIDs are used in such patients, the risk of developing aseptic meningitis is increased (see section "Adverse reactions").
This medicinal product contains less than 1 mmol (23 mg) sodium/dose, i.e. essentially 'sodium-free'.
Use during pregnancy or breastfeeding
Pregnancy period
Inhibition of prostaglandin synthesis may adversely affect pregnancy and/or embryo/foetal development. Epidemiological data indicate an increased risk of miscarriage and of cardiac malformations and gastroschisis after the use of prostaglandin synthesis inhibitors in early pregnancy. The absolute risk of cardiovascular malformations has increased from less than 1% to approximately 1.5%. The risk is thought to increase with increasing dose and duration of treatment. In animals, administration of a prostaglandin synthesis inhibitor has been shown to result in increased pre- and post-implantation losses and embryo/foetal lethality. In addition, an increased incidence of various malformations, including cardiovascular, has been reported in animals treated with a prostaglandin synthesis inhibitor during organogenesis.
From the 20th week of pregnancy, the use of tenoxicam may cause oligohydramnios due to fetal renal dysfunction. Renal impairment may occur almost immediately after initiation of treatment and is usually reversible after discontinuation of tenoxicam. In addition, there have been reports of narrowing of the ductus arteriosus following treatment in the second trimester of pregnancy, which resolved after discontinuation of treatment. Therefore, tenoxicam should not be administered during the first and second trimesters of pregnancy unless clearly necessary.
If tenoxicam is used by a woman attempting to conceive, or during the first and second trimesters of pregnancy, the lowest possible dose should be used for the shortest duration possible. Antenatal monitoring for oligohydramnios and narrowing of the ductus arteriosus should be considered after administration of tenoxicam for several days, starting from the 20th week of pregnancy. Oxyliten should be discontinued if signs of oligohydramnios or narrowing of the ductus arteriosus are observed.
During the third trimester of pregnancy, all prostaglandin synthesis inhibitors may have the following effects:
on the fruit:
cardiopulmonary toxicity (premature narrowing/closure of the ductus arteriosus and pulmonary hypertension);
renal dysfunction, which may progress to renal failure with oligohydramnios (see above);
on the mother and newborn, at the end of pregnancy:
possible prolongation of bleeding time; antiplatelet effect, which may be observed even at very low doses;
inhibition of uterine contractions, leading to delayed or prolonged labor.
Therefore, tenoxicam is contraindicated during the third trimester of pregnancy.
Breastfeeding period
Limited data from studies of NSAIDs suggest that very small amounts of the drugs may pass into breast milk. The use of NSAIDs during breastfeeding should be avoided if possible.
There is no information on the excretion of tenoxicam in human milk. Animal studies suggest that significant levels of the drug may be achieved.
Fertility
See section "Special warnings and precautions for use" for information on the effect on female fertility.
Ability to influence reaction speed when driving vehicles or other mechanisms
Patients who develop adverse reactions that may affect the ability to drive or use machines, such as vertigo, dizziness, drowsiness, fatigue or visual disturbances, should refrain from driving or using machines.
Method of administration and doses
Adverse reactions to tenoxicam can be minimized by using the lowest effective dose for the shortest duration necessary to control symptoms (see section "Special warnings and precautions for use").
Adults
The drug is intended for intravenous and intramuscular use.
The recommended dose of the drug is 20 mg once a day for the first 1-2 days of treatment, then you should switch to taking tablets, which should be taken daily at the same time. Before use, the contents of the vial must be dissolved in 2 ml of water for injection, which is included in the drug package. After complete dissolution of the lyophilisate, the solution should be used immediately.
The recommended doses of the drug should not be exceeded, since the use of higher doses does not always achieve a more pronounced therapeutic effect, and the risk of adverse reactions increases.
The duration of treatment with tenoxicam for acute musculoskeletal disorders usually does not exceed 7 days. In exceptional cases, the duration of therapy may be extended to 14 days.
Oxyliten, like other NSAIDs, should be used with special caution in elderly patients. They have an increased risk of developing adverse reactions and are more likely to receive concomitant medications or have impaired renal, hepatic, or cardiovascular function. If necessary, the drug should be used in elderly patients at the lowest effective dose for the shortest possible period of time. Such patients should be carefully monitored for gastrointestinal bleeding during NSAID therapy.
Patients with renal and/or hepatic impairment
| Creatinine clearance | Dosage |
| More than 25 ml/min | Under the supervision of a physician without correction of the dosage regimen (see section "Peculiarities of use") |
| Below 25 ml/min | Insufficient data to make a dosage recommendation |
The drug should be used with caution in cases of low albumin concentrations (e.g. nephrotic syndrome) or high plasma bilirubin concentrations, as tenoxicam is highly bound to plasma proteins.
There are insufficient data to make dosing recommendations for tenoxicam in patients with hepatic impairment.
Children.
There are insufficient data to make recommendations for the use of tenoxicam in children.
Overdose
Symptoms: There have been no reports of severe cases of overdose with tenoxicam. Symptoms of NSAID overdose include headache, nausea, vomiting, epigastric pain, gastrointestinal bleeding, occasionally diarrhea, disorientation, agitation, coma, drowsiness, dizziness, tinnitus, weakness, sometimes convulsions. Significant renal or hepatic failure is possible in severe poisonings.
Treatment. If necessary, symptomatic therapy should be carried out. Adequate hydration should be maintained, liver and kidney function should be monitored. The patient should be under medical supervision for at least 4 hours after overdose. In case of frequent or prolonged convulsions, intravenous diazepam should be administered. H2-antagonists may be useful. Other measures should be carried out as indicated according to the clinical condition of the patient.
Side effects
In most patients, adverse reactions are temporary and do not require discontinuation of treatment. The most common side effects are gastrointestinal.
Cardiovascular: Edema, hypertension and heart failure have been reported in association with NSAID treatment. Dyspnoea and palpitations have been reported rarely.
Clinical trials and epidemiological data suggest that the use of NSAIDs (particularly at high doses and in long-term use) may increase the risk of arterial thrombosis (e.g. myocardial infarction, stroke) (see section "Special warnings and precautions for use").
Skin and subcutaneous tissue disorders: Photosensitivity and bullous reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis (very rare), have been reported.
Visual disorders: Visual disorders (such as blurred vision and blurred vision) have been reported with an unknown frequency.
Gastrointestinal: The most common adverse reactions are related to the digestive tract. They include dyspepsia, nausea, vomiting, abdominal pain and discomfort, constipation, diarrhea, flatulence, indigestion, epigastric distress, melena, hematemesis, ulcerative stomatitis, anorexia, exacerbation of colitis and Crohn's disease (see section "Special instructions").
As with other NSAIDs, there is a risk of peptic ulceration, perforation or gastrointestinal bleeding, which can be fatal, especially in elderly patients (see section "Special warnings and precautions for use").
Gastritis was observed less frequently.
Pancreatitis has been reported very rarely.
Blood and lymphatic system disorders: Decreased haemoglobin levels have been observed, not associated with gastrointestinal bleeding. Anaemia, aplastic anaemia, haemolytic anaemia, thrombocytopenia and non-thrombocytopenic purpura, leukopenia, neutropenia and eosinophilia have been reported. Epistaxis has been uncommonly reported. Agranulocytosis has been reported rarely.
Hepatobiliary disorders: Liver function disorders. As with most NSAIDs, various changes in liver function parameters have been observed. Some patients may develop increases in serum transaminases during treatment. Although these reactions are rare, if persistent or worsening liver function test abnormalities occur, if clinical signs or symptoms of liver disease occur, or if systemic manifestations (e.g. eosinophilia, rash) occur, the drug should be discontinued. Hepatitis and jaundice have also been reported.
Metabolic disorders: Metabolic disorders such as hyperglycemia, weight gain or loss have been observed rarely.
Nervous system: malaise and tinnitus may occur.
Less frequent reports include aseptic meningitis (especially in patients with underlying autoimmune disorders such as systemic lupus erythematosus, mixed connective tissue disease) with symptoms such as neck stiffness, headache, nausea, vomiting, fever or disorientation, dizziness, malaise, fatigue and drowsiness.
Headache, insomnia, depression, nervousness, sleep disturbances and dizziness have been reported uncommonly. Somnolence and paraesthesia have been reported with an unknown frequency.
Psychiatric: Confusion and hallucinations have been reported with an unknown frequency.
Urinary system: Various forms of nephrotoxicity have been reported, including interstitial nephritis, nephrotic syndrome, and renal failure.
Reversible increases in blood urea nitrogen and creatinine have been reported (see section 4.4).
Adverse reaction reporting
Reporting of suspected adverse reactions after the approval of a medicinal product is important. This allows for continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system.
Expiration date
3 years.
Sterile!
Storage conditions
Store in the original packaging at a temperature not exceeding 25 °C.
Keep out of reach of children.
Incompatibility
Unknown.
Packaging
Lyophilisate for solution for injection 20 mg in vial No. 1 and solvent (water for injection) 2 ml in ampoule No. 1 in a cardboard box.
Vacation category
According to the recipe.
Producer
Anfarm Ellas S.A.
Location of the manufacturer and address of its place of business.
61st km of the Athens-Lamia National Road, Shimatari Viotias, 32009, Greece.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.