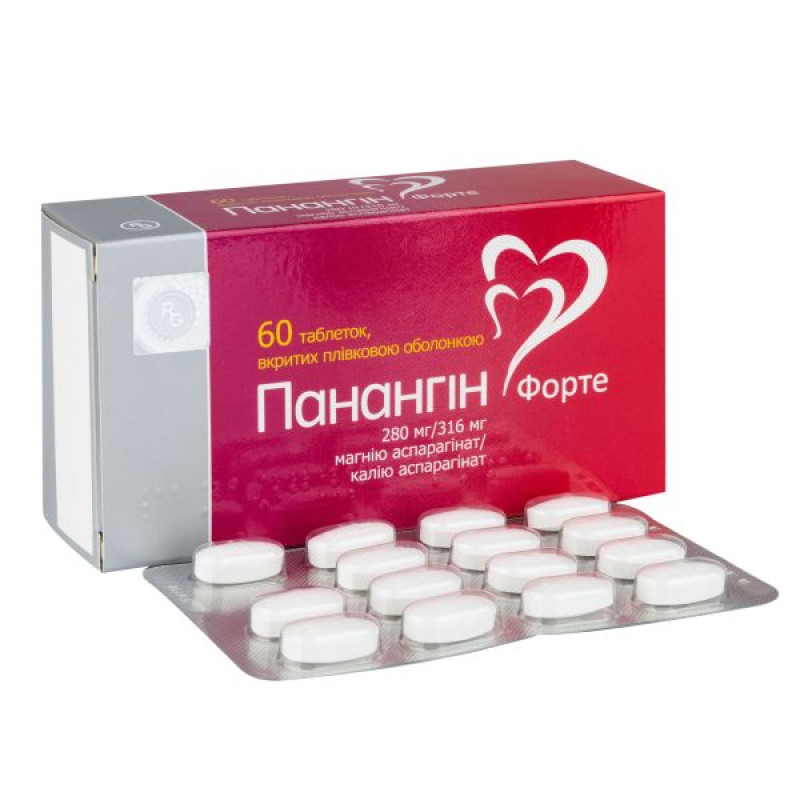
Panangin forte tablets No. 60

Instructions for Panangin forte tablets No. 60
Composition
active ingredients: magnesium asparaginate, potassium asparaginate;
1 film-coated tablet contains:
280 mg of magnesium asparaginate (as 350 mg of magnesium asparaginate tetrahydrate);
316 mg of potassium asparaginate (as 332.6 mg of potassium asparaginate hemihydrate);
excipients: colloidal anhydrous silicon dioxide; povidone K-30; potato starch; magnesium stearate; talc; corn starch;
film coating: macrogol 6000; titanium dioxide (E 171); butylated methacrylate copolymer (Eudragit E); talc.
Dosage form
Film-coated tablets.
Main physicochemical properties: oval biconvex tablets, film-coated, white or almost white in color, with a slightly shiny and slightly uneven surface, almost odorless, embossed with "A83" on one side.
Pharmacotherapeutic group
Mineral substances. Preparations of other mineral substances. ATX code A12C X.
Pharmacological properties
Pharmacodynamics
Potassium and magnesium ions are important intracellular cations that play a significant role in the work of a number of enzymes, in the process of binding macromolecules to subcellular elements and in the mechanism of muscle contraction at the molecular level. The ratio of extracellular and intracellular concentrations of potassium, calcium, sodium and magnesium ions affects the contractile ability of the myocardium. Aspartate as an endogenous substance is a suitable ion carrier, has a pronounced affinity for cells, its salts are subject to dissociation only to a small extent. As a result, the ions penetrate into the intracellular space in the form of complex compounds. Magnesium aspartate and potassium aspartate improve myocardial metabolism. Insufficient potassium and magnesium content in the body increases the risk of developing arterial hypertension, atherosclerotic lesions of the coronary vessels, heart rhythm disturbances, and myocardial pathology.
Pharmacokinetics
Magnesium
The total magnesium ion store in a human body weighing 70 kg is on average 24 g (1000 mmol); more than 60% of magnesium is in bone tissue and about 40% in skeletal muscles and other tissues. About 1% of the total magnesium ion store in the body is in the extracellular fluid, mainly in blood serum. In healthy adults, the magnesium content in blood serum is in the range of 0.7–1.10 mmol/L.
The recommended dietary intake of magnesium for men is 350 mg per day and for women, 280 mg per day. Magnesium needs increase during pregnancy and breastfeeding.
Magnesium is absorbed from the gastrointestinal tract by active transport. The kidneys are the main regulator of magnesium balance in the body. 3-5% of ionized magnesium is excreted by the kidneys.
Increased urine volume (e.g., with highly effective loop diuretic therapy) leads to increased excretion of ionized magnesium. If magnesium absorption in the small intestine is reduced, subsequent hypomagnesemia leads to decreased excretion (< 0.5 mmol/day).
Potassium
The total body potassium ion store in a 70 kg person is on average 140 g (3570 mmol). It is slightly lower in women than in men and decreases slightly with age. 2% of the total body potassium ion store is extracellular, while the remaining 98% is intracellular.
The optimal dietary intake of potassium is 3–4 g (75–100 mmol) per day. The main route of potassium excretion is renal (about 90% of potassium is excreted by the kidneys daily). The remaining 10% is excreted through the gastrointestinal tract. Thus, the kidneys are responsible for long-term potassium homeostasis, as well as for serum potassium. In the short term, blood potassium is also regulated by the exchange of potassium between intracellular and extracellular spaces.
Indication
Additional therapy for chronic heart diseases (heart failure, patients in the post-infarction period) and heart rhythm disorders (primarily ventricular arrhythmias), on the recommendation of a doctor.
Additional therapy when treating digitalis with drugs, as recommended by a doctor.
As a dietary supplement to increase magnesium and potassium levels in the body.
Contraindication
Hypersensitivity to the active substances or to any of the excipients of the drug.
Acute or chronic renal failure.
Addison's disease.
Third degree atrioventricular block.
Cardiogenic shock (blood pressure < 90 mm Hg).
Interaction with other medicinal products and other types of interactions
Drug interaction studies with potassium asparaginate and magnesium asparaginate have not been conducted. According to the scientific literature, potassium and magnesium may interact with some drugs. Concomitant use with potassium-sparing diuretics, angiotensin-converting enzyme inhibitors, beta-blockers, cyclosporine, heparin, nonsteroidal anti-inflammatory drugs may lead to hyperkalemia.
Tetracyclines used internally, iron salts and sodium fluoride inhibit the absorption of potassium asparaginate and magnesium asparaginate from the gastrointestinal tract. An interval of at least 3 hours is required between taking the above-mentioned drugs and the drug Panangin Forte.
Application features
Panangin Forte, as a drug containing potassium and magnesium, should be used with caution in patients with myasthenia gravis; in conditions that may lead to hyperkalemia, such as decreased renal function, acute dehydration, widespread tissue damage, in particular severe burns. In this category of patients, it is recommended to regularly examine the concentration of electrolytes in the blood serum.
Panangin Forte, film-coated tablets, should not be prescribed to patients with gastroduodenal ulcers or obstruction.
Use during pregnancy or breastfeeding
To date, there is no data on any harmful effects of using Panangin Forte in this category of patients.
The ability to influence the reaction speed when driving vehicles or using other mechanisms
Panangin Forte does not affect the ability to drive vehicles and other mechanisms.
Method of administration and doses
Dosage
Adults
The recommended daily dose is 1 tablet 3 times a day.
The maximum daily dose is 1 tablet 3 times a day.
The course of treatment is determined by the doctor.
Method of application
For internal use.
Gastric juice may reduce the effectiveness of the drug, so it is recommended to take Panangin Forte after meals.
Children.
The safety and efficacy of Panangin Forte film-coated tablets in children and adolescents have not been established. The drug is not recommended for use in this group of patients.
Overdose
There is no information on overdose with drugs containing potassium asparaginate and magnesium asparaginate, even when taking large doses.
Given the ability of the kidneys to excrete large amounts of potassium from the body, increasing the dose of the drug may lead to hyperkalemia only if it is associated with acute or severe impairment of potassium excretion.
The therapeutic index of magnesium is wide, and in the absence of renal failure, severe side effects are extremely rare.
According to scientific data, taking magnesium supplements orally may cause minor side effects, such as diarrhea.
Large doses of Panangin Forte may cause an increase in the frequency of bowel movements due to the magnesium content.
Symptoms of hyperkalemia: general weakness, paresthesias, bradycardia, paralysis. Extremely high plasma potassium concentrations can lead to death from cardiac depression, arrhythmia, or cardiac arrest.
Symptoms of hypermagnesemia: nausea, vomiting, drowsiness, hypotension, bradycardia, weakness, slurred speech, double vision. At very high plasma concentrations of magnesium, hyporeflexia, muscle paralysis, respiratory arrest and cardiac arrest may develop.
Treatment.
In case of overdose, the drug should be discontinued and, if necessary, symptomatic treatment should be carried out (intravenous calcium chloride, dialysis if necessary).
Side effects
On the part of the gastrointestinal tract: when using large doses of the drug, an increase in the frequency of bowel movements is possible.
According to some reports, nausea, vomiting, and abdominal pain may occur.
Expiration date
3 years.
Storage conditions
Store at a temperature not exceeding 25 ºС.
Keep out of reach of children.
Packaging
15 tablets in a blister.
2, 4 or 6 blisters in a cardboard box.
Vacation category
Without a prescription.
Producer
JSC "Gedeon Richter".
LLC "Gedeon Richter Poland".
Location of the manufacturer and address of its place of business.
H-1103, Budapest, Demrei Street 19-21, Hungary.
5, Kn. J. Ponyatowskiego Street, Grodzisk Mazowiecki, 05-825, Poland.
Applicant
JSC "Gedeon Richter".
Location of the applicant.
H-1103, Budapest, Demrei Street 19-21, Hungary.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.