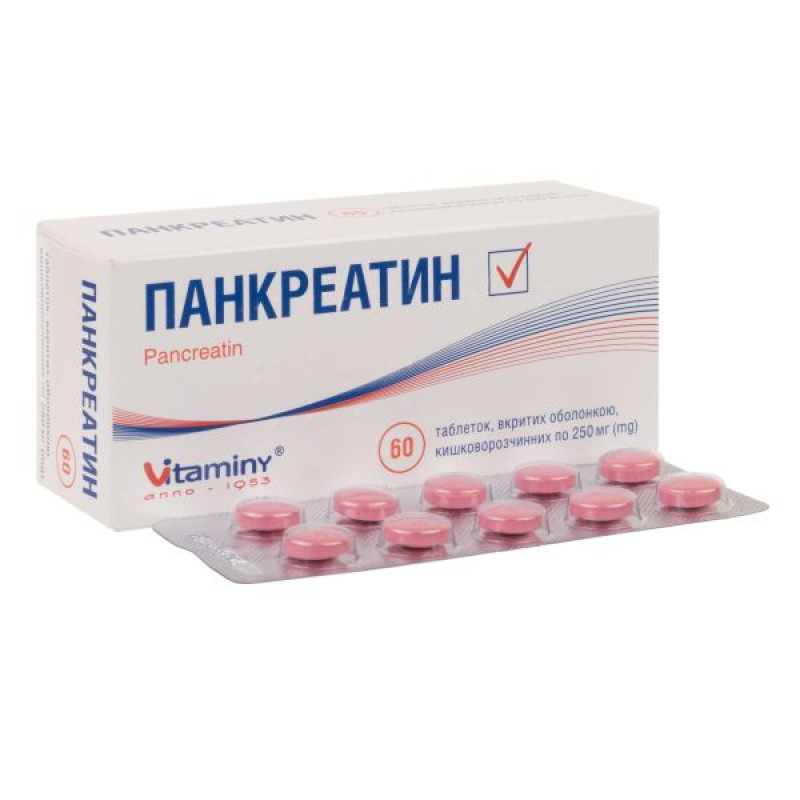
Pancreatin enteric-coated tablets 250 mg blister No. 60

Instructions Pancreatin enteric-coated tablets 250 mg blister No. 60
Composition
active ingredient: pancreatin;
1 tablet contains 250 mg of pancreatin, which corresponds to an activity of at least 1000 amylolytic U Ph. Eur., 1200 lipolytic U Ph. Eur., 80 proteolytic U Ph. Eur.;
excipients: lactose, monohydrate; povidone; calcium stearate; acrylic pink 93O34055 [mixture of substances: methacrylate copolymer (type C), talc, titanium dioxide (E 171), triethyl citrate, carmine (E 120), colloidal anhydrous silica, sodium bicarbonate, sodium lauryl sulfate, Ponceau 4R (E 124), indigo carmine (E 132)].
Dosage form
The film-coated tablets are enteric-coated.
Main physicochemical properties: round tablets, coated, with a smooth, biconvex surface, from light pink to dark pink in color, the presence of a specific odor is allowed. When broken, when viewed under a magnifying glass, a core surrounded by one continuous layer is visible.
Pharmacotherapeutic group
Digestive aids, including enzymes. Polyenzyme preparations.
ATX code A09A A02.
Pharmacological properties
Pharmacodynamics
Enzyme preparation from the pancreas of cattle and pigs. Pancreatic enzymes included in the preparation: lipase, a-amylase, trypsin, chymotrypsin - facilitate the process of digestion of proteins, carbohydrates and fats in food, contributing to a more complete absorption of nutrients in the small intestine.
Pharmacokinetics
Due to the acid-resistant coating, the enzymes are not inactivated by the hydrochloric acid of the stomach. The dissolution of the shell and the release of enzymes begins in the duodenum. The enzymes are poorly absorbed in the digestive tract, acting in the intestinal lumen.
Indication
Dyspepsia; simultaneous consumption of vegetable, fatty and unusual foods that are difficult to digest; flatulence associated with the above-mentioned disorders; acceleration of the passage of food in the intestines of a functional nature.
Contraindication
Individual hypersensitivity to pancreatin or other components of the drug. Acute pancreatitis, chronic pancreatitis in the acute phase, intestinal obstruction.
Interaction with other medicinal products and other types of interactions
When pancreatin is used simultaneously with antithrombotic agents, vitamin K antagonists and acetylsalicylic acid, the effect of these drugs is reduced. The effectiveness of non-selective inhibitors of neuronal monoamine reuptake is also reduced when used simultaneously with pancreatin.
When used simultaneously with M-cholinoblockers, the anticholinergic effect is enhanced.
When using the drug, it is possible to reduce the absorption of iron and folic acid, and reduce the hypoglycemic effect of acarbose.
Simultaneous use of antacids containing calcium carbonate and/or magnesium hydroxide, with tannin, alcohol-containing agents may lead to a decrease in the effectiveness of pancreatin.
Application features
Intestinal obstruction – if symptoms suggestive of this condition are present, the possibility of intestinal strictures should be considered. It is recommended to monitor any unusual symptoms, especially when taking more than 10,000 lipase units/kg/day.
To avoid the formation of uric acid stones, the uric acid content in the urine should be monitored.
The drug contains active enzymes that can damage the oral mucosa, so the tablets should be swallowed whole, without chewing.
With prolonged use, iron preparations should be prescribed simultaneously.
When taking the drug before meals, it should be washed down with alkaline liquids (for example, Borjomi mineral water or 0.5–1% sodium bicarbonate solution).
The drug contains lactose, therefore, if the patient has been diagnosed with intolerance to some sugars, you should consult a doctor before taking this drug.
The dye Ponceau 4R (E 124) in the preparation may cause allergic reactions.
Use during pregnancy or breastfeeding
Pancreatin can be used during pregnancy or breastfeeding only as prescribed by a doctor in cases where the expected benefit to the mother outweighs the potential risk to the fetus/child.
Ability to influence reaction speed when driving vehicles or other mechanisms
Does not affect.
Method of administration and doses
Adults and children over 6 years of age should take 1–2 tablets orally, during or immediately after meals, without chewing, with plenty of liquid.
Depending on the type of food consumed or the severity of indigestion, take 2–4 tablets during meals for enzyme replacement therapy.
The dose of the drug and the duration of treatment are determined by the nature of the disease and are set individually by the doctor.
Children
The drug can be used in children aged 6 years and over.
Overdose
Symptoms: hyperuricemia, hyperuricosuria; in children - constipation.
Treatment: drug withdrawal, adequate hydration, symptomatic therapy.
Adverse reactions
On the part of the digestive system: diarrhea, feeling of discomfort in the epigastric region of the stomach, nausea, vomiting, flatulence, change in the nature of stools, constipation, intestinal obstruction.
On the part of the immune system: hypersensitivity reactions are possible (including rash, itching, sneezing, lacrimation, bronchospasm, dyspnea, urticaria, anaphylactic reactions, angioedema).
Cardiovascular system: tachycardia.
General disorders: feeling hot, general weakness.
Expiration date
2 years.
Storage conditions
Store in the original packaging at a temperature not exceeding 25 °C.
Keep out of reach of children.
Packaging
10 tablets in a blister, No. 10×1 or No. 10×2, or No. 10×5, or No. 10×6 blisters in a cardboard pack.
Vacation category
Without a prescription.
Producer
JSC "VITAMINS".
Location of the manufacturer and its business address.
Ukraine, 20300, Cherkasy region, Uman city, Uspenska st., 31.
Applicant.
JSC "VITAMINS".
Location of the applicant.
Ukraine, 20300, Cherkasy region, Uman city, Uspenska st., 31.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.