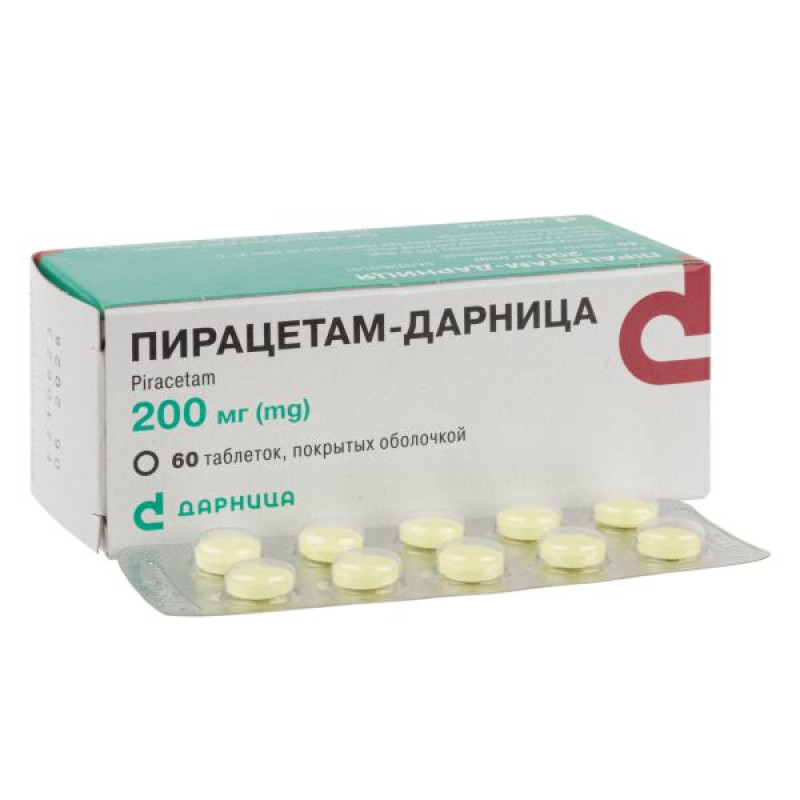
Piracetam-Darnitsa film-coated tablets 200 mg No. 60
Instructions for Piracetam-Darnitsa film-coated tablets 200 mg No. 60
Composition
active ingredient: piracetam;
1 tablet contains piracetam 200 mg;
excipients: heavy magnesium carbonate, potato starch, povidone, calcium stearate, hypromellose, macrogol 4000, titanium dioxide (E 171), quinoline yellow (E 104).
Dosage form
Film-coated tablets.
Main physicochemical properties: film-coated tablets, from light yellow to yellow or yellow with a reddish tint, round in shape with a biconvex surface. Two layers are visible on the cross-section.
Pharmacotherapeutic group
Psychostimulants and nootropics. Piracetam. ATX code N06B X03.
Pharmacological properties
Pharmacodynamics.
Piracetam is a nootropic that acts on the brain, improving cognitive processes such as learning ability, memory, attention, and mental performance. Piracetam affects the central nervous system in various ways: by changing the speed of propagation of excitation in the brain, improving metabolic processes in nerve cells, improving microcirculation, affecting the rheological characteristics of the blood and not causing a vasodilator effect.
Improves the connection between the cerebral hemispheres and synaptic conduction in neocortical structures. Piracetam inhibits platelet aggregation and restores the elasticity of the erythrocyte membrane, reduces erythrocyte adhesion. Piracetam has a protective and restorative effect in impaired brain function due to hypoxia and intoxication. Piracetam reduces the severity and duration of vestibular nystagmus.
Pharmacokinetics.
After oral administration, piracetam is rapidly and almost completely absorbed, with peak concentrations achieved 1 hour after administration. Bioavailability is almost 100% after a single dose of 2 g. The volume of distribution of piracetam is approximately 0.6 l/kg. The half-life of the drug from blood plasma is 4−5 hours and 6−8 hours from cerebrospinal fluid, which is prolonged in renal failure. It does not bind to plasma proteins and is not metabolized in the body. 80−100% of piracetam is excreted unchanged by the kidneys by renal filtration. The renal clearance of piracetam in healthy volunteers is 86 ml/min. The pharmacokinetics of piracetam do not change in patients with hepatic insufficiency. Piracetam penetrates the blood-brain and placental barriers and membranes used in hemodialysis. In animal studies, piracetam selectively accumulates in the tissues of the cerebral cortex, mainly in the frontal, parietal and occipital lobes, in the cerebellum and basal ganglia.
Indication
In adults:
symptomatic treatment of pathological conditions accompanied by memory loss and cognitive disorders, with the exception of diagnosed dementia;
Treatment of cortical myoclonus: as a monotherapy or as part of complex therapy.
Contraindication
Individual hypersensitivity to piracetam or pyrrolidone derivatives, as well as to other components of the drug.
Acute cerebrovascular accident (hemorrhagic stroke).
End-stage renal failure (creatinine clearance less than 20 ml/min).
Huntington's cholera.
Interaction with other medicinal products and other types of interactions
Pharmacokinetic interactions
The possibility of changing the pharmacodynamics of piracetam under the influence of other drugs is low, since 90% of piracetam is excreted unchanged in the urine.
In vitro, piracetam does not inhibit cytochrome P450 isoforms CYP1A2, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1 and 4A9/11 at concentrations of 142, 426, 1422 μg/ml.
At a concentration of 1422 μg/ml, a slight inhibition of CYP2A6 (21%) and ZA4/5 (11%). However, the Ki level of these two CYP isomers is sufficient above 1422 μg/ml. Therefore, metabolic interactions with drugs that are biotransformed by these enzymes are unlikely.
Thyroid hormones
When used simultaneously with thyroid hormones (T3+T4), increased irritability, disorientation, and sleep disturbances are possible.
Acenocoumarol
Clinical studies have shown that in patients with severe recurrent thrombosis, the use of piracetam in high doses (9.6 g/day) did not require a change in the dosage of acenocoumarol to achieve a prothrombin time (IT) of 2.5−3.5, but with its simultaneous use, a significant decrease in the level of platelet aggregation, fibrinogen levels, von Willebrand factors [coagulation activity (VIII: C); ristocetin cofactor (VIII: vW: Rco) and plasma protein (VIII: vW: Ag;)], blood viscosity and blood plasma was observed.
Antiepileptic drugs
The use of piracetam at a dose of 20 mg per day daily for 4 weeks did not change the concentration-level curve and maximum concentration of antiepileptic drugs (carbamazepine, phenytoin, phenobarbital, sodium valproate) in patients with epilepsy.
Alcohol
Concomitant administration with alcohol does not affect the concentration of piracetam in the blood serum, and the level of alcohol concentration in the blood serum does not change with a single dose of 1.6 g of piracetam.
In elderly people, piracetam enhances the effect of antianginal drugs and increases the effectiveness of antidepressants.
Application features
Effect on platelet aggregation.
Due to the fact that piracetam reduces platelet aggregation, the drug should be prescribed with caution to patients with impaired hemostasis; conditions that may be accompanied by bleeding (gastrointestinal ulcer); during major surgical operations (including dental operations); patients with symptoms of severe bleeding; patients with a history of hemorrhagic stroke; patients using anticoagulants, platelet antiaggregants, including low doses of acetylsalicylic acid.
Kidney dysfunction.
The drug is excreted by the kidneys, so special attention should be paid to patients with renal insufficiency.
Elderly patients.
With long-term therapy for elderly patients, regular monitoring of renal function is recommended; if necessary, the dose should be adjusted depending on the results of the creatinine clearance study.
Interruption of application.
When treating patients with cortical myoclonus, abrupt discontinuation of treatment should be avoided due to the risk of generalization of myoclonus or the occurrence of seizures.
The drug penetrates the filter membranes of hemodialysis machines.
Important information about excipients.
This medicinal product contains sodium. Caution should be exercised when used in patients on a controlled sodium diet.
Use during pregnancy or breastfeeding
Do not use the medicine during pregnancy or breastfeeding.
Ability to influence reaction speed when driving vehicles or other mechanisms
Caution should be exercised when driving or operating other machinery due to the possibility of developing adverse reactions from the central nervous system.
Method of administration and doses
The drug should be taken orally before or during meals. The tablets should be washed down with liquid (water or juice). The duration of treatment and the selection of an individual dose depend on the severity of the patient's condition and the speed of the reverse dynamics of the clinical picture of the disease.
Adult use
Treatment of conditions accompanied by memory impairment and cognitive disorders.
The initial daily dose is 4.8 g during the first week of treatment. The dose should be divided into 2-3 doses. The maintenance dose is 2.4 g per day (divided into 2-3 doses). Subsequently, a gradual dose reduction of 1.2 g per day is possible.
Treatment of cortical myoclonus.
The initial dose is 24 g for 3 days. If during this time the desired therapeutic effect is not achieved, the drug should be continued in the same dosage (24 g/day) for up to 7 days. If the therapeutic effect is weak or absent, the drug should be continued in the same dose for up to 7 days. If the desired therapeutic effect is not obtained on the 7th day of treatment, piracetam treatment should be discontinued. If the therapeutic effect has been achieved, then starting from the day when a stable improvement is achieved, the drug dose should be reduced by 1.2 g every 2 days until the manifestations of cortical myoclonus appear again. This will make it possible to establish the average effective dose.
The daily dose should be divided into 2-3 doses. Treatment with other antimyoclonic agents should be maintained at previously prescribed doses. Treatment should be continued until the symptoms of the disease disappear. To prevent deterioration of the patient's condition, the drug should not be abruptly discontinued. The dose should be gradually reduced by 1-2 g every 2-3 days. Repeated courses of treatment should be prescribed every 6 months, adjusting the dose depending on the patient's condition, until the symptoms of the disease disappear or decrease.
Use in elderly patients
Dose adjustment is recommended for elderly patients with known or suspected renal impairment. During long-term treatment, creatinine clearance should be monitored in such patients, if necessary, to ensure adequate dose adjustment.
Dosage in patients with renal impairment
Since the drug is excreted from the body by the kidneys, caution should be exercised when treating patients with renal insufficiency.
The increase in half-life is directly related to the deterioration of renal function and creatinine clearance. This also applies to elderly patients, in whom creatinine clearance is age-dependent. The interval between doses should be adjusted based on renal function.
The dose calculation should be based on the patient's estimated creatinine clearance using the formula:
[140 − age (in years)] × body weight (in kg)
Kcr= ─ (× 0.85 for women)
72 × Plasma creatinine (mg/dL)
Prescribe treatment to such patients depending on the severity of renal failure, adhering to the following recommendations:
| Degree of renal failure | Creatinine clearance (ml/min) | Dosage |
| − | > 80 | Usual dose divided into 2 or 4 doses |
| Light | 50−79 | 2/3 of the usual dose in 2-3 doses |
| Moderate | 30−49 | 1/3 of the usual dose in 2 doses |
| Severe | < 30 | 1/6 of the usual dose once | Terminal stage | − | Contraindicated |
No dose adjustment is required in patients with hepatic impairment. In case of diagnosed or suspected hepatic or renal impairment, dose adjustment should be carried out as indicated in the section “Dosage in patients with renal impairment”.
Children.
The medicine should not be used in children.
Overdose
Symptoms: increased side effects of the drug. When taking 75 g of piracetam orally, dyspeptic symptoms such as bloody diarrhea and abdominal pain were noted. Symptoms of overdose were observed with oral administration of the drug in a dose of 75 g.
Treatment is symptomatic. Immediately after a significant oral overdose, gastric lavage or induce vomiting is necessary. There is no specific antidote, hemodialysis can be used (excretion of 50-60% of piracetam).
Side effects
Side effects are classified by organ system and frequency of occurrence.
The frequency is defined as follows: very common (≥ 1/10), common (≥ 1/100, < 1/10), uncommon (≥ 1/1000, ≤ 1/100), rare (≥ 1/10000, ≤ 1/1000), very rare (≤ 1/10000), isolated cases (frequency cannot be estimated from the available data).
Adverse reactions reported during post-marketing surveillance are listed below by system organ class.
From the vestibular system.
Rare: vertigo.
From the gastrointestinal tract.
Isolated cases: anorexia, abdominal pain, upper abdominal pain, nausea, diarrhea, vomiting, constipation.
From the side of metabolism.
Common: weight gain.
From the nervous system:
Common: hyperkinesia.
Uncommon: drowsiness.
Isolated cases: extrapyramidal disorders, ataxia, tremor, balance disorders, dizziness, headache, agitation, irritability, sleep disorders, insomnia, increased frequency of epileptic seizures, convulsions.
From the psyche:
Common: nervousness.
Uncommon: depression.
Isolated cases: increased excitability, confusion, anxiety, confusion, hallucinations.
From the cardiovascular system.
Isolated cases: worsening of angina pectoris, arterial hypertension.
From the blood and lymphatic system.
Isolated cases: thrombophlebitis, hemorrhagic disorders.
From the immune system.
Rare: hypersensitivity reactions, including anaphylaxis.
On the skin and subcutaneous tissue.
Isolated cases: angioedema, dermatitis, itching, rash, urticaria.
From the reproductive system.
Isolated cases: increased sexual activity.
General disorders.
Isolated cases: hyperthermia, asthenia.
Reporting of suspected adverse reactions.
Reporting suspected adverse reactions after the marketing authorisation of a medicinal product is an important procedure. It allows for continued monitoring of the benefit-risk balance of the medicinal product in question. Healthcare professionals should report any suspected adverse reactions via the national reporting system.
Expiration date
5 years.
Storage conditions
Store in the original packaging at a temperature not exceeding 25 °C.
Keep out of reach of children.
Packaging
10 tablets in a contour blister pack; 6 contour blister packs in a pack.
Vacation category
According to the recipe.
Producer
PrJSC "Pharmaceutical Company "Darnitsa".
Address
Ukraine, 02093, Kyiv, Boryspilska St., 13.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.