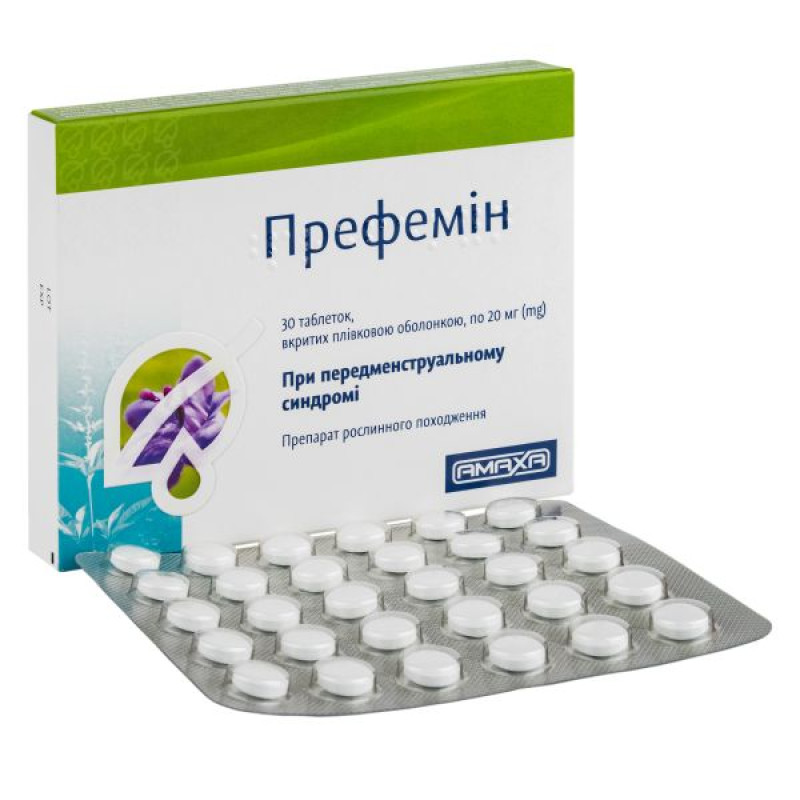
Prefemin film-coated tablets 20 mg blister No. 30
Instructions Prefemin film-coated tablets 20 mg blister No. 30
Composition
active ingredient: 1 tablet contains 20 mg of dry native extract of common agni fruit (Fructis Agni casti) (6–12:1), extractant – ethanol 60% (m/m);
Excipients: microcrystalline cellulose; lactose monohydrate; magnesium stearate; colloidal anhydrous silica; hypromellose; macrogol 400; titanium dioxide (E 171); macrogol 20000; propylene glycol.
Dosage form
Film-coated tablets.
Main physicochemical properties: round biconvex white tablets with a fragrant odor.
Pharmacotherapeutic group
Means used in gynecology.
ATX code G02C X03.
Pharmacological properties
Pharmacodynamics
The drug is a herbal medicine for the treatment of premenstrual syndrome.
The drug has a normalizing effect on the level of sex hormones. The dopaminergic effects of the drug cause a decrease in the production of prolactin, i.e. eliminate hyperprolactinemia. An increased concentration of prolactin disrupts the secretion of gonadotropins, as a result of which disturbances may occur during follicle maturation, ovulation and in the corpus luteum stage, which subsequently leads to an imbalance between estradiol and progesterone. This imbalance between sex hormones causes menstrual disorders. Unlike estrogens and other hormones, prolactin also has a direct stimulating effect on proliferative processes in the mammary glands, enhancing the formation of connective tissue and expanding the milk ducts. A decrease in the level of prolactin leads to the reverse development of pathological processes in the mammary glands and stops the pain syndrome. Rhythmic production and normalization of the ratio of gonadotropic hormones contribute to the normalization of the second phase of the menstrual cycle.
Pharmacokinetics
No data available.
Indication
Herbal medicine for the treatment of premenstrual syndrome.
Contraindication
Hypersensitivity to common thorn fruit or to any of the excipients of the drug.
Interaction with other medicinal products and other types of interactions
Interactions with dopamine agonists, dopamine antagonists, estrogens, and antiestrogens cannot be ruled out due to the possible dopaminergic and estrogenic effects of common primrose.
Application features
Patients who have or have had estrogen-dependent tumors, as well as patients who are taking dopamine agonists, dopamine antagonists, estrogens, and antiestrogens, should consult a doctor before using products containing Agnus Castus.
If you have a history of pituitary disorders, you should consult a doctor before using the drug, as the fruits of common thorn affect the hypothalamic-pituitary system.
In patients with prolactin-secreting pituitary tumors, the use of common thorn fruit may mask the symptoms of the tumor.
There are no clinical data on the use in patients with severe renal or hepatic impairment.
If symptoms worsen while using the drug, you should consult a doctor.
This medicinal product contains lactose. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicinal product.
Prefemin harmonizes the hormonal balance in the female body, the harmonization process can last up to 3 months. During this time, menstrual cycle disorders are possible.
Use during pregnancy or breastfeeding
Not recommended for use during pregnancy or breastfeeding (may cause decreased lactation).
The ability to influence the reaction speed when driving or working with other mechanisms
If dizziness occurs during treatment with the drug, you should refrain from driving or operating other mechanisms.
Method of administration and doses
The drug should be taken 1 tablet once a day. The film-coated tablets should be swallowed whole with plenty of water. It is advisable to take the tablets at about the same time, for example, in the morning or before going to bed, regardless of meals. The treatment lasts for 3 months without a break during menstruation. Even after the condition improves, the treatment should be continued for several more weeks. If the symptoms do not go away after taking the drug for 3 months, you should consult a doctor.
Children
Due to insufficient data, the drug is not recommended for use in children.
Overdose
In case of overdose, treatment is symptomatic.
Side effects
The assessment of adverse reactions is based on the following criteria: very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1000 to <100), rare (≥1/10000 to <1/1000), very rare (<1/10000), unknown (cannot be estimated from the available data).
Immune system: serious allergic reactions with facial swelling, shortness of breath and difficulty swallowing - frequency unknown.
Gastrointestinal disorders: gastrointestinal disorders (nausea, abdominal pain) - frequency unknown.
Skin and subcutaneous tissue disorders: allergic skin reactions (rash, urticaria), acne - frequency unknown.
Reproductive system and breast disorders: menstrual disorders - frequency unknown.
In some cases, symptoms of premenstrual syndrome may worsen after the first dose of Prefemin.
If any reactions occur, discontinue use of the drug and consult a doctor.
Expiration date
3 years.
Storage conditions
Store at a temperature not exceeding 25 °C in the original packaging. Keep out of the reach of children.
Packaging
30 film-coated tablets in a blister; 1 or 3 blisters in a cardboard box.
Vacation category
Without a prescription.
Producer
Max Zeller Söhne AG
Max Zeller Sоhne AG
Location of the manufacturer and address of its place of business.
Seeblickstrasse 4, 8590 Romanshorn, Switzerland
Seeblickstrasse 4, 8590 Romanshorn, Switzerland
Applicant
Amaxa Ltd.
Amaxa Ltd
Location of the applicant.
31 John Islip Street, London SW1P 4FE, United Kingdom
31 John Islip Street, London SW1P 4FE, United Kingdom
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.