Pumpan tablets No. 48
Instructions for Pumpan tablets No. 48
Warehouse
active ingredient: 1 tablet contains: Crataegus D1 74.8 mg; Arnica D6 37.2 mg; Kalium carbonicum D6 37.2 mg; Digitalis D12 37.2 mg; Convallaria D12 37.2 mg;
excipients: lactose monohydrate, potato starch, magnesium stearate.
Dosage form
Pills.
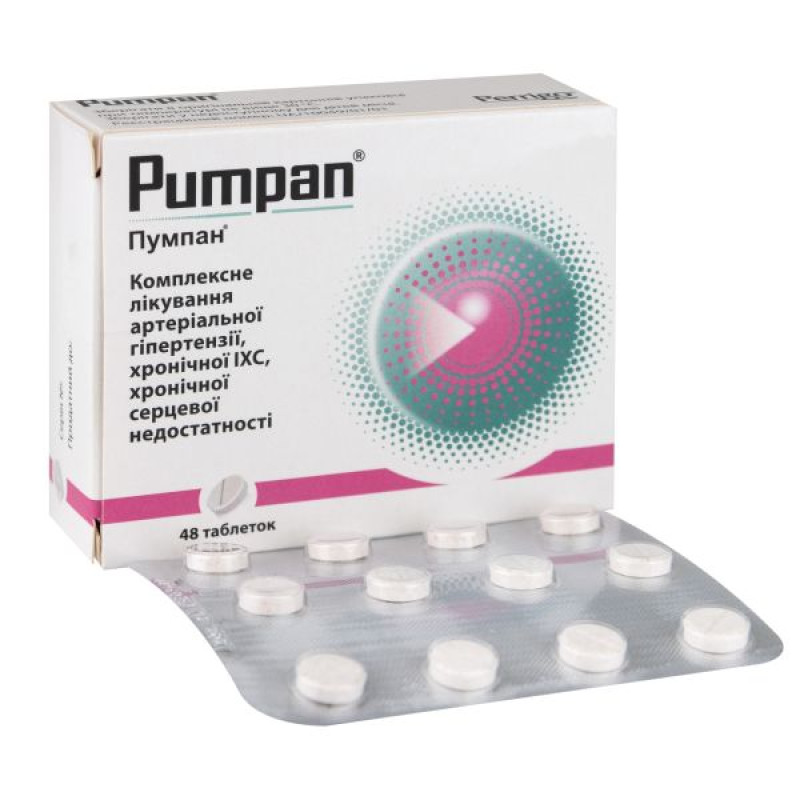
Main physicochemical properties: round, flat tablets of almost white color with a slight orange tint, with possible inclusions, with a dividing line.
Pharmacotherapeutic group
A complex homeopathic preparation.
Pharmacological properties
Pharmacodynamics.
The drug improves blood circulation, metabolic processes and nutrition of the heart muscle, increases the efficiency and economy of the heart, helps to improve the contractile and pumping function of the myocardium. Improves lipid metabolism without additional use of hypolipidemic drugs. Promotes normalization of high blood pressure, strengthens the walls of blood vessels and has an antisclerotic, antiarrhythmic effect. Improves the rheological properties of blood.
In complex treatment, Pumpan® reduces the frequency and intensity of angina attacks, strengthens the cardiovascular system, increases the heart's resistance to physical and psycho-emotional stress, prevents further development and exacerbation of heart disease. Reduces the need and allows you to reduce the intake or doses of other cardiovascular drugs.
Improves the quality of life and adaptive capabilities of people suffering from cardiovascular diseases.
Indication
In the complex therapy of chronic ischemic heart disease, arterial hypertension, dystrophic changes in the myocardium, heart rhythm disorders, chronic heart failure.
Neurocirculatory dystonia of cardiac or mixed type.
Contraindication
Hypersensitivity (allergy) to any component of the drug.
Interaction with other medicinal products and other types of interactions
Clinically significant interactions of Pumpan® with other drugs have not been established. The drug can be combined with any drugs and treatment methods.
When using Pumpan® simultaneously with other cardiovascular drugs, the dose of basic therapy drugs can be reduced, but only under the supervision of a physician.
Application features
The tablet dosage form contains lactose, therefore patients with rare hereditary forms of galactose intolerance, lactase deficiency or glucose-galactose malabsorption syndrome should take this medicine instead of tablets.
Pumpan® should be used in the form of drops.
Since Pumpan® contains natural plant components, a slight change in the taste and color of the tablet may occur during storage, which does not reduce the effectiveness of the drug.
Use during pregnancy or breastfeeding
Information on any risk to the fetus and child from the use of the drug during pregnancy or breastfeeding has not yet been registered. The drug can be used during pregnancy or breastfeeding in cases where the expected benefit to the mother outweighs the possible risk to the fetus/child.
Ability to influence reaction speed when driving or using other mechanisms
The drug does not affect the ability to drive vehicles and mechanisms.
Method of administration and doses
Adults and children over 12 years of age: 1 tablet;
children aged 5-12 years: ½ tablet.
Take 2 times a day for 2-3 months.
When the condition stabilizes, it is possible to switch to a single dose (maintenance dose).
To achieve maximum effect, it is recommended to take Pumpan® between meals (30 minutes before or 1 hour after a meal). It is recommended to keep the tablet in the mouth until completely dissolved.
At the beginning of treatment, as well as in cases requiring rapid relief of symptoms, it is recommended to take the drug 4 times a day for the first two days, after which you should switch to the standard regimen of taking it 2 times a day.
Children
There is no experience with the use of the drug in children under 5 years of age.
Overdose
No cases of overdose have been identified.
Adverse reactions
In exceptional cases, allergic reactions are possible in individuals with hypersensitivity to any component of the drug.
Expiration date
5 years.
Storage conditions
Store in the original cardboard packaging at a temperature not exceeding 30 ° C. Keep out of the reach of children.
Packaging
12 tablets in a blister; 1 or 2, or 3, or 4 blisters in a cardboard box.
Vacation category
Without a prescription.
Producer
Richard Bittner AG, Austria.
Location of the manufacturer and its business address
Ossiacherstrasse 7, A-9560 Feldkirchen, Austria.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.