Rapid coronavirus test Testsealabs saliva antigen COVID-19 No. 1
PRINCIPLE OF OPERATION
The COVID-19 virus antigen saliva rapid test is a qualitative immunoassay based on a chromatographic immunological reaction on a membrane for the detection of SARS-CoV-2 virus antigen in saliva samples.
In this rapid test, anti-SARS-CoV-2-N antibodies are immobilized in the test line region of the test pen. After collecting a saliva sample, the test sample reacts with anti-SARS-CoV-2-N antibody particles contained in the test strip. This mixture migrates chromatographically along the length of the test strip and interacts with the immobilized anti-SARS-CoV-2-N antibody.
If the test specimen contains SARS-CoV-2 nucleocapsid protein, a colored line will appear in the test line region, indicating a positive result. If the test specimen does not contain SARS-CoV-2 nucleocapsid protein, no colored line will appear in the test line region, indicating a negative result. As an internal control, a colored line always appears in the control line region, indicating that the sample volume was sufficient and that it was absorbed by the membrane.
REAGENTS
This test contains one anti-SARS-CoV-2-N antibody as the capture reagent and another anti-SARS-CoV-2-N antibody as the detection reagent. Goat anti-mouse IgG antibodies are used in the control line system.
Reservation
- For in vitro diagnostic use only.
- Do not use after the expiration date.
- Do not use the test if the integrity of the packaging is damaged.
- The rapid test should be used within 30 minutes (min) after opening the foil package.
- It is not recommended to consume food, drinks, or smoke (including electronic cigarettes) during and at the test site.
- All specimens should be handled as if they contain infectious agents. Observe established microbiological precautions during all procedures and dispose of appropriately, in accordance with local regulations.
- Before beginning the test, it is important to carefully read the testing and interpretation instructions. Specimen collection is critical to the performance of the test. Failure to follow these procedures may result in inaccurate results.
- Use disposable gloves when handling samples.
- If a third party collects the sample, they should use personal protective equipment such as disposable gloves, a protective mask, and safety glasses.
- It should be noted that violations of storage conditions (from +4 °C to +30 °C) and environmental parameters during testing, namely excessive humidity (more than 90%) and deviations from the recommended temperature (below +15 °C and above +30 °C), may negatively affect the analysis results.
Storage conditions
The rapid test should be stored in its original packaging, sealed, at room temperature or in a refrigerator (at temperatures between +4°C and +30°C). The rapid test is stable until the expiration date indicated on the sealed package. The rapid test should remain in the sealed package until used. DO NOT FREEZE. Do not use the rapid test after the expiration date.
Equipment
| Package contents | Additional required materials |
Test pen 1 pc. Instructions for use 1 pc. | Timer Disposable gloves Protective mask Safety glasses |
Method of application
You should not eat, drink, or smoke (including e-cigarettes) for at least 30 minutes (min) before the test.
Before performing the analysis, the sealed package with the rapid test pen should be kept at room temperature for a sufficient time to bring it to room temperature (+15°C to +30°C).
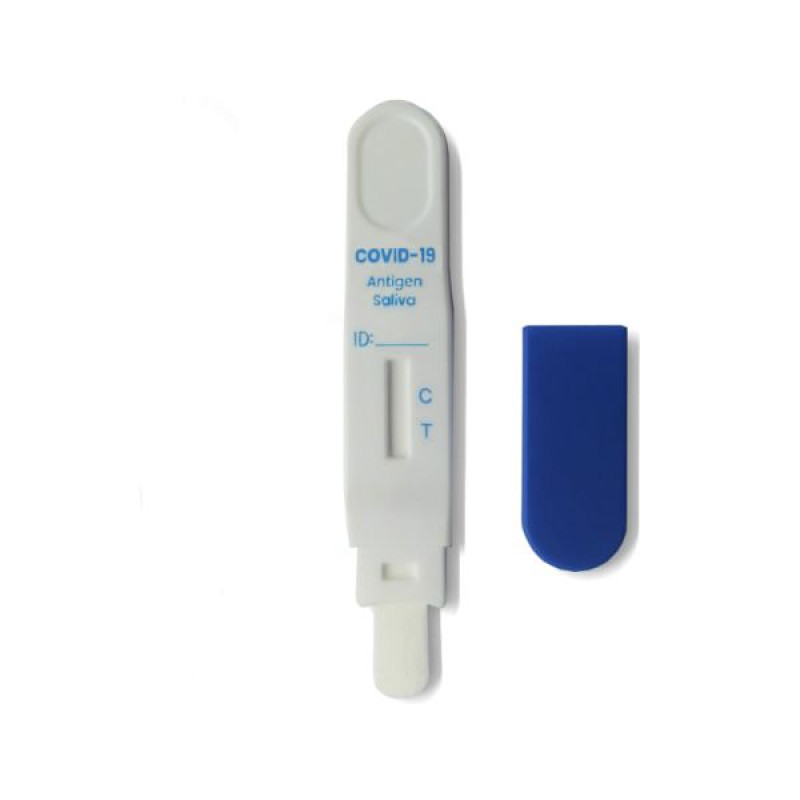
- Open the foil pouch and place the test pen on a clean and flat surface. The test pen should be used within 30 minutes (min) of opening.
- Remove the cap from the test pen and moisten the cotton tip of the test pen with saliva. To do this, place the end of the test pen with the cotton core in your mouth, directly under your tongue. Your tongue should be held on the cotton tip of the test pen; do not curl your tongue. The cotton tip of the test pen should be immersed in the saliva for 2 minutes (min) or until liquid appears in the viewing window of the test pen.
- After 2 minutes (min) or when liquid appears in the cassette viewing window, remove the test pen from your mouth, close the cap, and place it on a clean, flat surface.
- Start the timer. Check the result after 15 minutes (min).
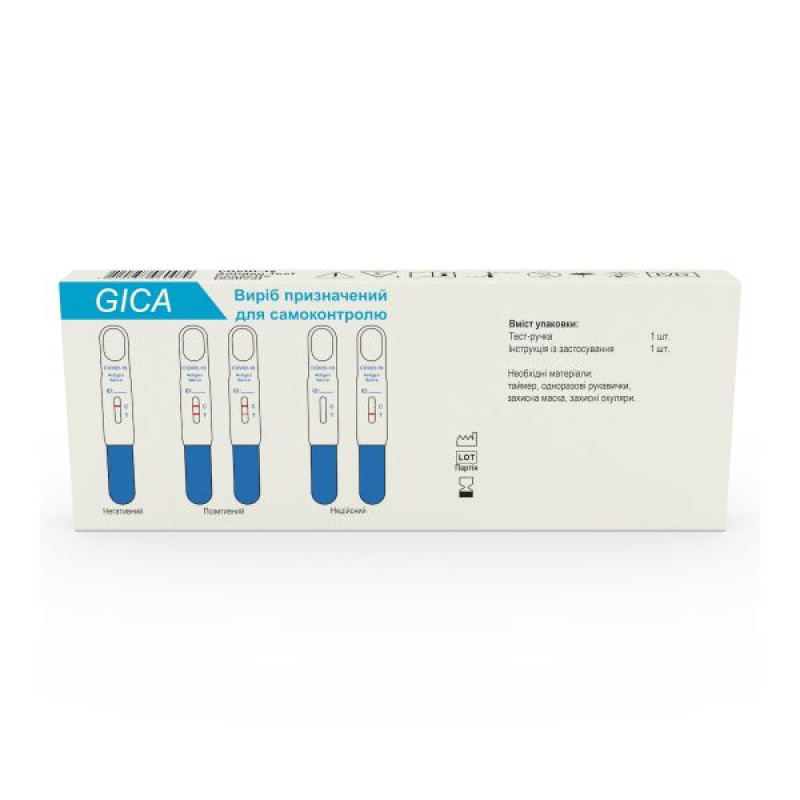
INTERPRETATION OF RESULTS
Positive: Two colored lines appear. One colored line should always appear in the control line region (C) and the other in the test line region (T).
If you receive a positive result, you should contact your doctor as soon as possible for help or instructions on further action.
NOTE: The intensity of the color in the test line region (T) may vary depending on the concentration of SARS-CoV-2 virus antigen present in the specimen. Accordingly, any shade of color in the test line region should be considered positive.
If you receive a negative result but have typical symptoms of COVID-19, you should contact your doctor as soon as possible for help or instructions on further actions.
Invalid: Control line does not appear. Insufficient sample volume or incorrect steps during the testing procedure are the most likely causes of a missing control line. Please re-read the instructions for use carefully and repeat the test with a new test.
If the problem persists, you should immediately stop using the test and contact your local distributor.
DO NOT MAKE ANY MEDICAL DECISIONS BASED ON THE TEST RESULT WITHOUT CONSULTING A DOCTOR!
QUALITY CONTROL
The test includes an internal procedural control. The colored line that appears in the control region (C) is the internal procedural control. It indicates that the volume of the test sample is sufficient and the procedure technique is correct.
LIMITATION
- Test results should not be used as the sole method for diagnosing SARS-CoV-2 infection. Additional molecular diagnostic and clinical parameters should be used to establish the diagnosis.
- This test detects both viable (live) and non-viable SARS-CoV-2 viruses. The performance of the test depends on the amount of virus (antigen) in the test sample and may or may not correlate with results obtained after virus isolation from the same sample.
- The result obtained during the test does not exclude the possibility of concomitant infection with other pathogens. It is recommended to consult a doctor for additional examinations and tests.
- A positive test result does not distinguish between SARS-CoV and SARS-CoV-2 viruses.
- The test should be performed no later than 1 hour (h) after opening the foil package, otherwise erroneous analysis results may occur.
- Positive test results do not distinguish between SARS-CoV and COVID-19 strains.
- Sampling for too short a time may give a negative result.
- A negative test result may occur if the antigen concentration in the test sample is below the detection limit of the test, especially in the first asymptomatic days after infection.
- A false negative result may occur if the test sample is collected improperly, or if it is further processed or transported.
- Negative results in patients with symptom onset more than seven days later should be considered presumptive and confirmed with another molecular test.
- If differentiation of specific viruses and strains of COVID-19 is required, additional testing should be performed in consultation with state or local health departments.
Producer
HANGZHOU TESTSEA BIOTECHNOLOGY CO., LTD.
3rd Floor, Building 6, No. 8-2 Keji Road, Yuhang District, Hangzhou, China
Authorized representative in Ukraine: LLC "ATIS PHARMA", 03022, Ukraine, Kyiv, Kozatska Street, building 122, office 100
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.