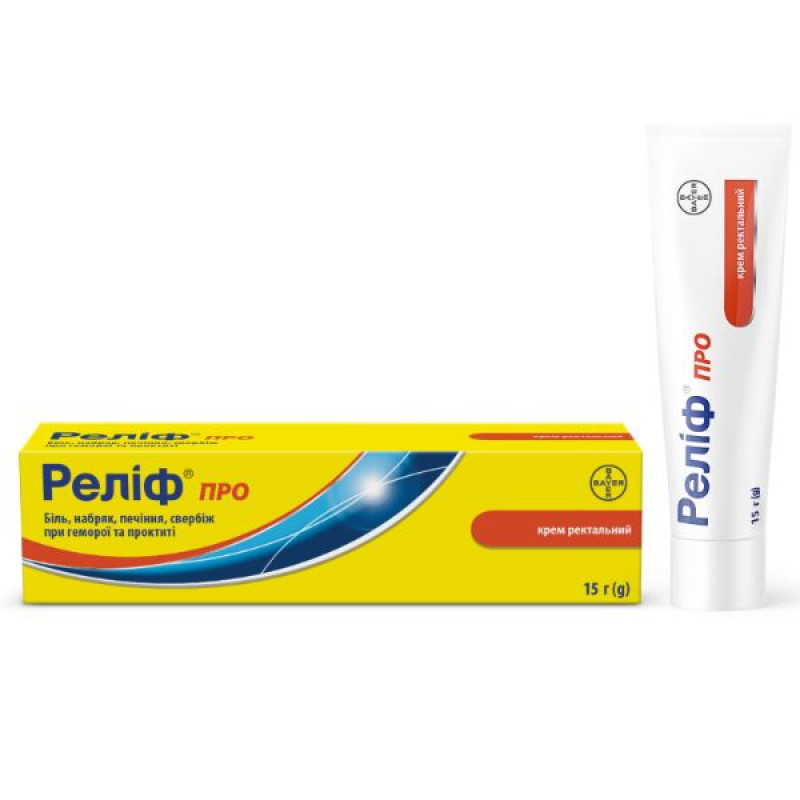
Relief pro rectal cream tube with applicator 15 g
Instructions for Relief rectal cream tube with applicator 15 g
Composition
active ingredients: 1 g of rectal cream contains 1 mg of fluocortolone pivalate and 20 mg of lidocaine hydrochloride (anhydrous);
excipients: polysorbate 60, sorbitan stearate, cetostearyl alcohol, mineral oil, white soft paraffin, disodium edetate, sodium dihydrogen phosphate dihydrate, sodium hydrogen phosphate dodecahydrate, benzyl alcohol, purified water.
Dosage form
Rectal cream.
Main physicochemical properties: white opaque cream.
Pharmacotherapeutic group
Topical agents for the treatment of hemorrhoids and anal fissures. Corticosteroids. Fluocortolone. ATC code C05AA08.
Pharmacological properties
Pharmacodynamics
Fluocortolone pivalate suppresses inflammatory and allergic skin reactions, and also alleviates subjective manifestations such as itching, burning sensation and pain; reduces capillary dilation, interstitial cell edema and tissue infiltration, inhibits capillary proliferation.
Lidocaine hydrochloride is a standard local anesthetic that has been used in medical practice for many years. Due to its analgesic effect, it is effective in the use of suppositories and ointments intended for the treatment of disorders associated with hemorrhoidal pathology. The reduction of pain and itching is associated with the inhibition of afferent nerve impulses.
Pharmacokinetics
After application of the cream to the rectal area in healthy male volunteers, the maximum systemic absorption was 15% of the dose of fluocortolone pivalate and 30% of the dose of lidocaine hydrochloride (active substances labeled with a radioactive isotope).
Indication
For symptomatic treatment of pain and inflammation in the case of:
- hemorrhoids;
- proctitis;
- eczema in the anus area.
Contraindication
The use of the drug Relief Pro is contraindicated in case of local infections in the treatment area, as well as in the presence of clear symptoms of the following pathological conditions in the relevant areas:
- specific skin lesions (syphilis, tuberculosis);
- chickenpox;
- reactions after vaccination;
- genital herpes;
- smallpox;
- other viral infections;
- primary bacterial or fungal infections;
- secondary skin infections in the absence of appropriate therapy.
Patients with hypersensitivity to other amide-type local anesthetics (e.g., bupivacaine, etidocaine, mepivacaine, and prilocaine).
Relief Pro should not be used in case of hypersensitivity to the active substances or to any of the excipients, for example to cetostearyl alcohol.
Special safety precautions
When using Relief Pro rectal cream in the genital area or in the anal area, excipients such as white soft paraffin and mineral oil may reduce the strength of condoms made of latex, thus compromising their reliability.
For fungal infections, additional special antifungal therapy is required.
Avoid contact with eyes. Wash hands thoroughly after application.
Cetostearyl alcohol may cause local skin reactions (contact dermatitis).
The use of topical preparations, especially for a long period, may cause sensitization. In this case, the use of these preparations should be discontinued and appropriate treatment should be initiated. If topical preparations are applied to large areas of the body, to damaged skin or under an airtight dressing, corticosteroids may be absorbed in quantities that may cause undesirable systemic effects. Do not apply under occlusive dressings in the perineum. Adrenal suppression may occur even without occlusion. Topical application of corticosteroids in large doses may result in the absorption of systemically active amounts of corticosteroids. There are reports in the scientific literature of the development of cataracts in patients using corticosteroids for a long period of time, therefore, in order to exclude manifestations of systemic effects of corticosteroids, one should be aware of the possible role of corticosteroids in the development of cataracts.
Use with caution in acute illnesses, debilitated patients, elderly patients; patients with severe heart failure, renal failure, and hepatic failure.
Interaction with other medicinal products and other types of interactions
Interaction studies have not been conducted. Patients taking antiarrhythmic drugs should use Relief Pro rectal cream with caution, as it contains lidocaine as an active ingredient.
Concomitant use with CYP3A inhibitors, including cobicistat in tablet form, is thought to increase the risk of systemic adverse effects. This combination should be avoided unless the benefit outweighs the risk of systemic adverse effects associated with corticosteroid use. In case of concomitant use, the patient should be monitored to ensure that there are no systemic adverse effects associated with corticosteroid use.
Use during pregnancy or breastfeeding
There are insufficient data on the use of Relief Pro rectal cream in pregnant women. Studies of glucocorticoids in animals have shown reproductive toxicity.
Some epidemiological studies suggest a possible increased risk of cleft palate in newborns whose mothers received systemic glucocorticoid treatment during the first trimester of pregnancy. Cleft palate is a rare condition, and although systemic glucocorticoid use is teratogenic, its use can only be considered a cause if the incidence increases to one or two per 1000 women who received the treatment during pregnancy.
Data on the topical use of glucocorticoids for the treatment of women during pregnancy are insufficient, but the risk can be considered low, since glucocorticoids have very low systemic bioavailability when applied topically.
In general, topical preparations containing glucocorticoids should not be used during the first trimester of pregnancy.
When prescribing treatment to pregnant and breastfeeding women, the clinical indications for the use of Relief Pro rectal cream should be carefully assessed, as well as the risk-benefit ratio. In particular, long-term use of the drug should be avoided.
Ability to influence reaction speed when driving vehicles or other mechanisms
No effect.
Method of administration and doses
Relief Pro rectal cream should be applied twice a day, morning and evening (approximately 1 gram per application). In the first days of treatment, the drug can be applied three times a day. After improvement, it is often sufficient to apply the drug once a day. It is recommended to apply Relief Pro rectal cream after defecation. Before applying the drug, the anal area should be carefully cleaned.
The total duration of treatment should not exceed 2 weeks.
Apply Relief Pro rectal cream on the tip of the finger in the area of the anus, overcoming the resistance of the sphincter with the tip of the finger. One dose of application on the tip of the finger is the amount obtained after a single squeeze from the tube and applied to the surface of the index finger of an adult. If it is necessary to introduce Relief Pro rectal cream into the rectum, screw the applicator onto the tube and insert the tip into the anus. A small amount of cream can also be introduced by lightly pressing the tube.
Do not use the applicator if it is damaged. Screw the applicator fully onto the tube. After each use, wipe the top of the applicator with a paper towel, remove any remaining cream from the middle of the applicator with cotton swabs, and wipe the applicator again with a paper towel. Rinse the applicator under warm water for 1 minute and dry it with a paper towel.
Children
Not recommended for use in children due to lack of data on safety and efficacy.
Overdose
The results of studies on the potential acute toxicity associated with the active ingredients of Relief Pro indicate that there is no risk of developing symptoms associated with acute toxicity that develops as a result of accidental overdose as a result of a single rectal application of the drug.
In case of accidental ingestion of the medicinal product (in case of ingestion of a few grams of cream), mainly systemic symptoms caused by the use of lidocaine hydrochloride are expected. Depending on the dose, these may manifest themselves in the form of severe cardiovascular disorders (decreased blood pressure, increased sweating, pallor of the skin, bradycardia, arrhythmia, depression of cardiac function, shock or in particularly severe cases - cardiac arrest) or reactions associated with disorders of the central nervous system (headache, dizziness, blurred vision, diplopia, tinnitus, drowsiness, numbness of the extremities, chills, anxiety, convulsions, dyspnea or in particularly severe cases - respiratory failure). Methemoglobinemia is possible.
Treatment of overdose includes close monitoring of vital signs, supportive measures to maintain oxygen levels, and symptomatic treatment of central nervous system and cardiovascular disorders, such as short-acting barbiturates, beta-sympathomimetics, and atropine. Dialysis is not effective.
Adverse reactions
Skin and subcutaneous tissue disorders, including allergic reactions: common: pain and burning sensation at the application site; uncommon: irritation at the application site.
After long-term therapy with Relief Pro cream (exceeding 4 weeks), there is a risk of developing local pathological skin conditions such as atrophy, striae or telangiectasia.
Adverse reactions due to lidocaine hydrochloride: anaphylactic reactions. The development of systemic adverse reactions is unlikely, since when using the drug, the entry of lidocaine into the systemic circulation is insignificant; systemic adverse reactions associated with lidocaine are identical in manifestations to those associated with local anesthetics of the amide group.
Expiration date
3 years.
After first opening, the shelf life is 4 weeks.
Storage conditions
Store out of the reach of children at a temperature not exceeding 30 °C.
Packaging
15 g or 30 g in a tube, 1 tube with an applicator in a cardboard pack with labeling in Ukrainian.
Vacation category
Without a prescription.
Producer
LEO Pharma Manufacturing Italy SRL.
Location of the manufacturer and its business address
Via E. Schering, 21, 20054, Segrate, Milan, Italy / Via E. Schering, 21, 20054, Segrate (MI), Italy.
Applicant
Bayer Consumer Care AG.
Applicant's location
Peter Merian-Strasse 84, 4052 Basel, Switzerland.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.