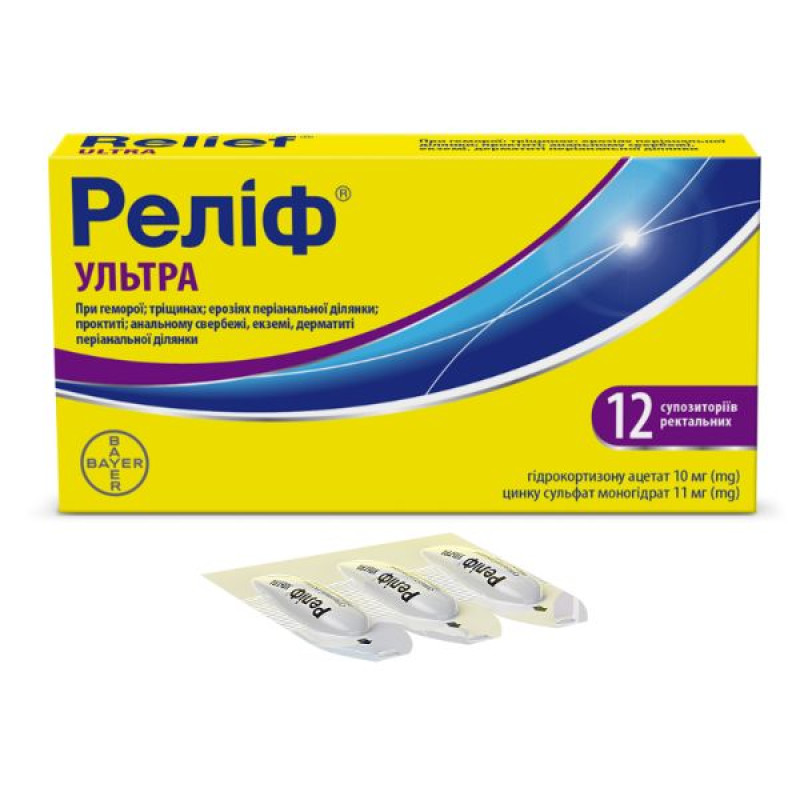
Relief Ultra rectal suppositories No. 12

Instructions for Relief Ultra rectal suppositories No. 12
Composition
active ingredients: 1 suppository contains hydrocortisone acetate 10 mg, zinc sulfate monohydrate 11 mg;
Excipients: shark liver oil, methyl parahydroxybenzoate (E 218), propyl parahydroxybenzoate (E 216), calcium hydrogen phosphate anhydrous, cocoa butter, magnesium stearate.
Dosage form
Rectal suppositories.
Main physicochemical properties: opaque suppositories from pale white to light yellow in color, torpedo-shaped, with a fishy odor.
Pharmacotherapeutic group
Means for the treatment of hemorrhoids and anal fissures for topical use. ATC code: C05A A.
Pharmacological properties
Pharmacodynamics
Hydrocortisone acetate is a glucocorticosteroid that, when applied topically, exhibits anti-inflammatory, antiallergic, vasoconstrictive, and antipruritic effects. It inhibits the release of inflammatory mediators and blocks the metabolism of arachidonic acid.
Zinc sulfate monohydrate promotes the healing of wounds and erosions, normalizes skin hydration.
Pharmacokinetics
When applied topically, the ingredients of the drug are excreted in mucus or in extremely small quantities are excreted in urine and bile. No manifestations of resorptive action are observed when using the drug in recommended doses. The ingredients of the drug do not penetrate into breast milk.
Indication
External and internal hemorrhoids, fissures, fistulas, ulcers, erosions of the perianal area and rectum, which are accompanied by pronounced inflammatory manifestations; proctitis, anal itching, eczema, dermatitis of the perianal area.
Contraindication
Increased individual sensitivity to any component of the drug (allergic reactions), specific (bacterial, fungal, viral, tuberculous) lesions of the anorectal area, neoplasms in the anus, thromboembolic disease.
Interaction with other medicinal products and other types of interactions
Use with caution during concomitant treatment with anticoagulants, hypoglycemic agents, barbiturates, diuretics, and cardiac glycosides.
Concomitant use with other corticosteroids (both topical and oral) may increase the likelihood of systemic effects.
Application features
In case of significant bloody discharge from the anus or if symptoms of the disease occur within 7 days of treatment, additional consultation with a proctologist is necessary. Accidental contact of the drug with the eyes should be avoided.
When using any topical steroids, the possibility of systemic absorption should be kept in mind.
Use during pregnancy or breastfeeding
Some data suggest that in rare cases there is a risk of cleft palate and intrauterine growth retardation, as well as suppression of the hypothalamic-pituitary-adrenal axis in the newborn. This medicine should not be used during the first trimester of pregnancy.
During pregnancy and breastfeeding, the drug can be used if, in the opinion of the doctor, the possible benefit of use for the woman outweighs the potential risk to the fetus.
Ability to influence reaction speed when driving vehicles or other mechanisms
Data is missing.
Method of administration and doses
Pre-wash the skin around the anus with warm water, clean the affected area with a damp soft cloth, and carefully dry it with toilet paper or a soft cloth. Before inserting the suppository, remove the protective plastic cover. Insert the suppository into the anus. Insert one suppository up to 4 times a day (at night, in the morning, and after each bowel movement). The duration of the treatment course should not exceed 7 days.
Children
The drug can be used in children over 12 years of age.
Overdose
Long-term use in large doses enhances resorption and increases the risk of developing systemic effects of hydrocortisone, such as menstrual irregularities, increased blood pressure, delayed wound healing, muscle weakness, insomnia, increased blood sugar levels, etc.
If the recommended single and daily doses of shark liver oil are significantly exceeded, a tendency to accelerated blood clotting may be observed.
In such cases, the use of the drug should be discontinued.
Accidental ingestion may cause gastrointestinal disorders (nausea, stomach pain).
Adverse reactions
Rarely, allergic reactions may occur, including hyperemia (redness), edema, itching; dryness of the mucous membrane.
Skin and subcutaneous tissue disorders: perianal dermatitis with or without skin atrophy; irritation, burning, rash, dry skin, pustular acne; "rebound effect", which may lead to steroid dependence; delayed wound healing.
Methyl parahydroxybenzoate and propyl parahydroxybenzoate may cause allergic reactions (possibly delayed) and in isolated cases bronchospasm.
Expiration date
2 years.
Storage conditions
Store out of the reach of children at a temperature not exceeding 25 °C.
Packaging
2 plastic strips of 6 suppositories in a cardboard box.
Vacation category
Without a prescription.
Producer
Istituto De Angeli Srl, Italy.
Manufacturer's location and business address
Localita' Prulli 103/c - 50066 Reggello (FL), Italy.
Applicant
Bayer Consumer Care AG, Switzerland.
Applicant's location
Peter Merian-Strasse 84, 4052 Basel, Switzerland.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.