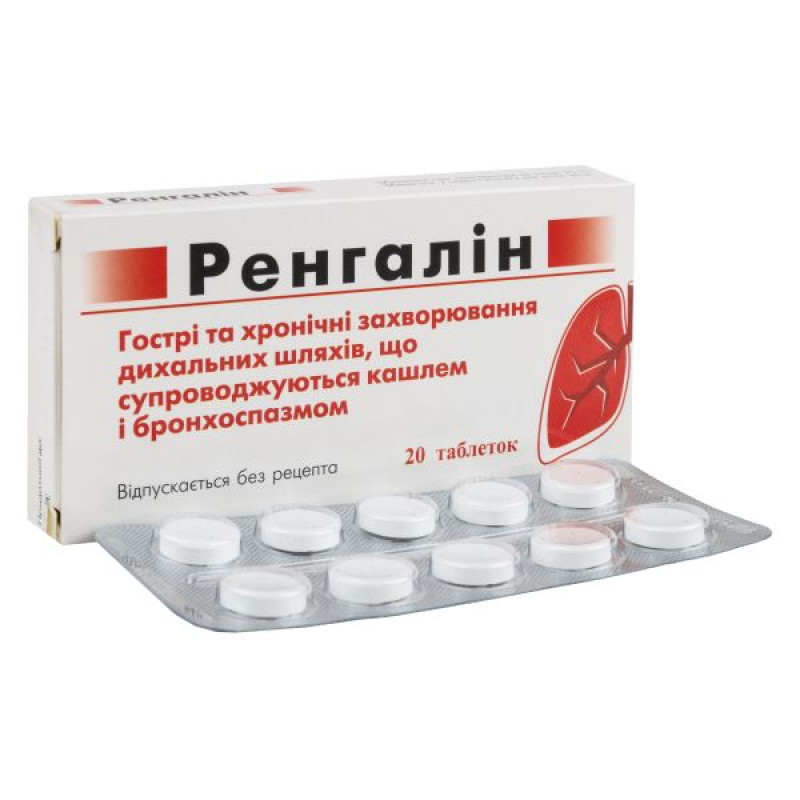
Rengalin lozenges No. 20

Instructions for Rengalin lozenges No. 20
Composition
Active ingredients: 1 tablet contains: affinity purified antibodies to bradykinin: a mixture of homeopathic dilutions C12, C30 and C50 - 6 mg; affinity purified antibodies to histamine: a mixture of homeopathic dilutions C12, C30 and C50 - 6 mg; affinity purified antibodies to morphine: a mixture of homeopathic dilutions C12, C30 and C50 - 6 mg;
Excipients: isomalt, microcrystalline cellulose, magnesium stearate, anhydrous citric acid, aspartame (E 951), sodium saccharin.
Dosage form
Pills.
Main physicochemical properties: flat-cylindrical tablets, with a score and a bevel, white to almost white in color. One flat side is scored, the other flat side is marked with the inscription RENGALIN.
Pharmacotherapeutic group
A complex homeopathic preparation.
Pharmacological properties
Pharmacodynamics.
It has been experimentally proven that the components of the drug modify the activity of the ligand-receptor interaction of endogenous regulators with the corresponding receptors: antibodies to morphine - with opiate receptors; antibodies to histamine - with H1-histamine receptors; antibodies to bradykinin - with B1-bradykinin receptors; while the combined use of the components leads to an increase in the antitussive effect.
In addition to the antitussive effect, the complex drug, due to the components included in its composition, has anti-inflammatory, anti-edematous, anti-allergic, antispasmodic (antibodies to histamine, antibodies to bradykinin) and analgesic effects (antibodies to morphine).
The drug, by modifying the histamine-dependent activation of H1 receptors and bradykinin-dependent activation of B2 receptors, selectively reduces the excitability of the cough center of the medulla oblongata, inhibits the central links of the cough reflex. By suppressing the centers of pain sensitivity in the thalamus, it blocks the transmission of pain impulses to the cerebral cortex. It suppresses the flow of pain impulses from the periphery due to a decrease in the release of tissue and plasma algogens (histamine, bradykinin, prostaglandins, etc.). Unlike narcotic analgesics, it does not cause respiratory depression, drug dependence, and does not have a narcotic or hypnotic effect.
Alleviates the symptoms of acute pharyngitis, laryngitis and bronchitis, reducing bronchospasm. Relieves systemic and local symptoms of allergic reactions by affecting the synthesis and release of histamine and bradykinin from mast cells.
Pharmacokinetics.
The sensitivity of modern physicochemical analysis methods (gas-liquid chromatography, high-performance liquid chromatography, chromatography-mass spectrometry) does not allow assessing the content of active components of a medicinal product in biological fluids, organs and tissues, which makes it technically impossible to study pharmacokinetics.
Indication
Acute and chronic diseases of the respiratory tract accompanied by cough and bronchospasm. Productive and unproductive cough in influenza and acute respiratory viral infections, acute pharyngitis, laryngotracheitis, acute obstructive laryngitis, chronic bronchitis and other infectious-inflammatory and allergic diseases of the upper and lower respiratory tract.
Contraindication
Increased individual sensitivity to the components of the drug.
Contraindicated in children (under 18 years of age).
Interaction with other medicinal products and other types of interactions
No cases of incompatibility with other drugs have been registered.
Application features
Information for patients with phenylketonuria: the drug contains aspartame (E 951) in an amount of 0.41 mg per 1 tablet.
Aspartame (E 951) is a derivative of phenylalanine, which is dangerous for people with phenylketonuria.
Use during pregnancy or breastfeeding
There are no data on the safety of the drug during pregnancy or breastfeeding. If the drug is necessary, the doctor should consider the risk-benefit ratio.
Ability to influence reaction speed when driving vehicles or other mechanisms
The drug does not affect the ability to drive vehicles and other potentially dangerous mechanisms.
Method of administration and doses
The drug is taken orally, 1 tablet per dose (do not take the drug with food). Keep the tablet in your mouth (preferably without chewing or swallowing) until completely dissolved.
Take 1–2 tablets 3 times a day.
Depending on the severity of the condition, the frequency of administration can be increased to 4–6 times a day in the first 3 days (only as prescribed by a doctor).
Children
Do not use in children (under 18 years of age).
Overdose
In case of accidental overdose, dyspeptic manifestations caused by the components included in the drug are possible.
Adverse reactions
Reactions of increased individual sensitivity to the components of the drug are possible.
If these side effects worsen, or you notice any other side effects not listed in the instructions, please inform your doctor.
Expiration date
3 years.
Do not use after the expiration date.
Storage conditions
Store at a temperature not exceeding 25 °C.
Keep out of reach of children.
Packaging
10 tablets in a blister; 2 blisters in a cardboard box.
Vacation category
Without a prescription.
Producer
Santonica CJSC.
Location of the manufacturer and its business address
St. Waveryu 134B, Kaunas, Kaunas sam., LT-46353, Lithuania.
Applicant
LLC "Materia Medica-Ukraine".
Applicant's location
Ukraine, 03062, Kyiv, Nyvska St., building 20,
Tel.: +380 (44) 400-90-78.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.