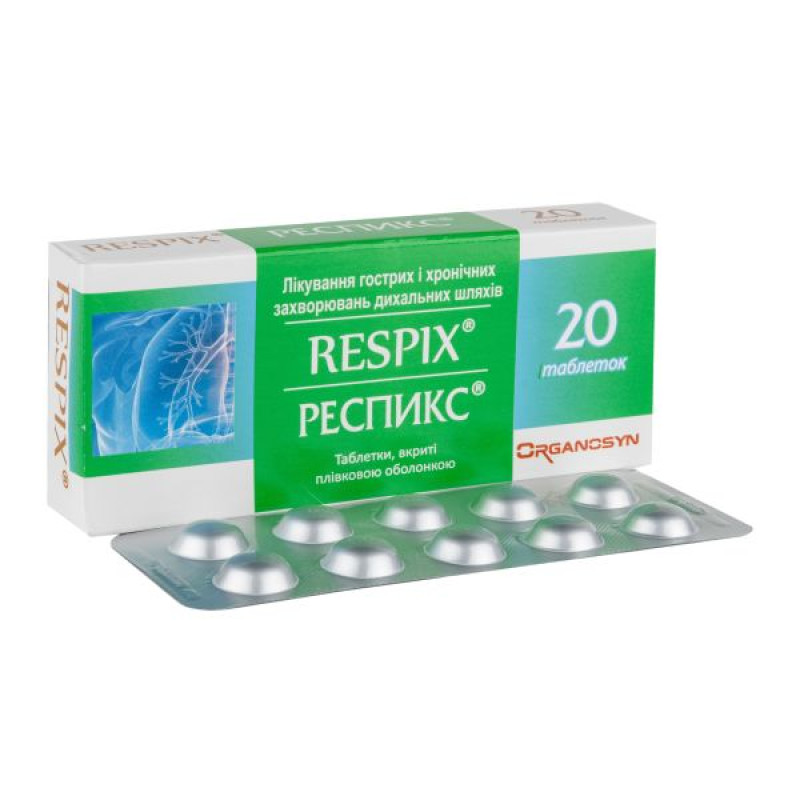
Respiks film-coated tablets blister pack No. 20

Instructions for Respiks film-coated tablets, blister pack No. 20
Composition
active ingredients: ambroxol hydrochloride, acetylcysteine.
1 tablet contains acetylcysteine 200 mg, ambroxol hydrochloride 30 mg;
Excipients: microcrystalline cellulose, povidone, magnesium stearate, lactose monohydrate, sodium starch glycolate, colloidal anhydrous silica, hypromellose, talc, polyethylene glycol 6000, titanium dioxide (E 171), sunset yellow FCF dye (E 110).
Dosage form
Film-coated tablets.
Main physicochemical properties: round, biconvex, film-coated tablets, orange in color.
Pharmacotherapeutic group
Drugs used for coughs and colds. Mucolytics. Combinations. ATX code R05C B10.
Pharmacological properties
Pharmacodynamics.
Ambroxol is a mucolytic and expectorant, has a pronounced expectorant, anti-inflammatory, immunomodulatory, antioxidant and minor antitussive effect. Stimulates serous cells of the glands of the bronchial mucosa, increases the amount of mucous secretion and thus changes the disturbed ratio of serous and mucous components. This normalizes the rheological parameters of sputum, reducing its viscosity and adhesive properties. Directly stimulates the mobile activity of the ciliated epithelium of the bronchi, prevents its clumping and improves mucociliary evacuation of sputum. Ambroxol increases the content of surfactant in the lungs, and also prevents its destruction by pneumocytes. Ambroxol does not cause bronchoobstruction, but on the contrary, improves the function of external respiration. It has been proven that the drug reduces the hyperreactivity of the bronchial muscles in patients with asthma. Ambroxol has anti-inflammatory effect, antioxidant properties, stimulates local immunity and renewal of the natural surfactant layer. After taking Ambroxol, patients' complaints of cough and sputum are significantly reduced according to the intensity of treatment.
Acetylcysteine is a mucolytic and expectorant. Due to the free sulfhydryl group, it breaks the disulfide bonds of mucopolysaccharides of sputum, which reduces the viscosity of bronchial secretions. Increases mucociliary clearance. Has an antioxidant effect by binding free radicals. Increases the synthesis of glutathione, which is an important factor in detoxification; due to this property, acetylcysteine is used to treat acute poisoning with paracetamol, phenols, aldehydes and other substances.
Pharmacokinetics.
Ambroxol.
Absorption.
Absorption of ambroxol hydrochloride is rapid and fairly complete, with linearity in the therapeutic range. Peak plasma levels are reached after 1-2.5 hours after oral administration of the immediate-release dosage forms.
Distribution.
The distribution of ambroxol hydrochloride is rapid and pronounced, with the highest concentration of the active substance in the lungs. The volume of distribution is approximately 552 l. In the blood plasma in the therapeutic range, approximately 90% of ambroxol hydrochloride is bound to proteins.
Metabolism.
Ambroxol hydrochloride is metabolized mainly in the liver by glucuronidation and cleavage to dibromanthranilic acid (approximately 10% of the dose). CYP3A4 is responsible for the metabolism of ambroxol hydrochloride to dibromanthranilic acid.
Breeding.
About 30% of the dose is eliminated by first-pass metabolism. After 3 days of oral administration, about 6% of the dose is excreted in the urine as unchanged drug and about 26% as conjugated drug. The elimination half-life is approximately 10 hours.
Acetylcysteine.
Absorption.
Acetylcysteine is completely absorbed after oral administration. Due to metabolism in the intestinal wall and the "first-pass" effect, bioavailability is very low (approximately 10%). Maximum plasma concentrations are reached 1-3 hours after administration and remain high for 24 hours.
Distribution.
The volume of distribution of acetylcysteine is from 0.33 to 0.47 l/kg. Protein binding is about 50% 4 hours after administration and decreases to 20% after 12 hours.
Metabolism.
Acetylcysteine is metabolized in the intestinal walls and liver.
Breeding.
Acetylcysteine is excreted by the kidneys in the form of inactive metabolites (inorganic sulfates, diacetylcysteine). The half-life is approximately 1 hour. In case of reduced liver function, the half-life is extended to 8 hours.
Indication
Treatment of acute and chronic respiratory diseases accompanied by impaired bronchial secretion and secretion evacuation (including acute and chronic bronchitis, chronic obstructive pulmonary diseases, pneumonia, bronchiectasis, bronchial asthma, cystic fibrosis, laryngitis, tracheitis).
Use during preparation for bronchoscopy and after the examination.
Contraindication
Hypersensitivity to the active substances or to other components of the drug.
Gastric and duodenal ulcer in the acute stage.
Hemoptysis.
Pulmonary hemorrhage.
Interaction with other medicinal products and other types of interactions
The simultaneous use of the drug and cough suppressants may lead to excessive mucus accumulation due to suppression of the cough reflex. Therefore, such a combination is possible only after a careful assessment by the doctor of the ratio of the expected benefit and the possible risk of use.
Ambroxol, which is part of the drug, when used simultaneously increases the concentration of the antibiotics amoxicillin, cefuroxime, erythromycin, and doxycycline in sputum and bronchopulmonary secretions.
With simultaneous use of acetylcysteine with cephalosporins (except cefixime and loracarbef), tetracyclines (except doxycycline), amphotericin B, aminoglycosides, a decrease in the activity of the above drugs is possible. Therefore, the interval between the use of these drugs and acetylcysteine should be at least 2 hours.
Concomitant administration of nitroglycerin and acetylcysteine may lead to an increase in the vasodilatory effect of nitroglycerin.
Synergism of acetylcysteine with bronchodilators is noted.
The use of acetylcysteine may alter the results of colorimetric salicylate quantification and urine ketone determination.
Application features
Use the drug with caution:
– patients with a history of gastric and duodenal ulcers, especially in the case of concomitant use of other medications that irritate the gastric mucosa;
– patients with impaired kidney function or severe liver disease (the interval between administrations should be increased or the dose reduced);
– patients with impaired bronchial motility and increased mucus secretion (for example, in a rare disease such as primary ciliary dyskinesia);
- patients with bronchial asthma (with bronchial conductivity control). In case of bronchospasm, the drug should be discontinued.
There have been a few reports of severe skin reactions, Stevens-Johnson syndrome and toxic epidermal necrolysis (Lyell's syndrome), associated with the use of expectorants such as ambroxol. These were mostly explained by the severity of the underlying disease in the patients and/or the concomitant use of another drug. Also, in the initial stage of Stevens-Johnson syndrome or Lyell's syndrome, patients may present with non-specific, flu-like symptoms such as fever, aches, rhinitis, cough and sore throat. Symptomatic treatment with cough and cold medicines may be mistakenly used for such non-specific, flu-like symptoms. Therefore, if new skin or mucous membrane lesions appear, patients should stop taking the drug and seek medical attention immediately.
The use of the drug causes liquefaction of bronchial secretions. If the patient is unable to cough up sputum effectively, postural drainage and bronchoaspiration are necessary.
The medicine contains lactose and should not be used in people with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption.
Use during pregnancy or breastfeeding
The drug is not recommended for use during pregnancy.
Ambroxol and acetylcysteine pass into breast milk. Breastfeeding should be discontinued if the drug is used.
Ability to influence reaction speed when driving vehicles or other mechanisms
There is no data on the effect of the drug on the reaction speed when driving vehicles or other mechanisms.
Method of administration and doses
Adults and children over 12 years of age: the recommended dose is 1 tablet 3 times a day. The tablets should be taken after meals and washed down with sufficient liquid. The drug should not be used for more than 4-5 days without consulting a doctor.
Children.
The drug should be used in children over 12 years of age.
Overdose
Ambroxol was well tolerated after parenteral administration in doses up to 15 mg/kg/day and after oral administration up to 25 mg/kg/day. In case of overdose with ambroxol, no signs of severe intoxication were observed. Cases of short-term restlessness and diarrhea, hypersalivation have been reported. According to preclinical studies, overdose may lead to hypersalivation, nausea, vomiting and decreased blood pressure. Overdose with acetylcysteine may cause
nausea, vomiting, diarrhea. Children are at risk of hypersecretion.
Treatment: symptomatic and supportive therapy.
Adverse reactions
Cardiovascular system: tachycardia, arterial hypotension.
From the nervous system: headache.
On the part of the organs of hearing: ringing in the ears.
Respiratory system: rhinorrhea, dry airways, bronchospasm (predominantly in patients with bronchial hyperreactivity associated with bronchial asthma).
On the part of the digestive tract: dry mouth, drooling, heartburn, nausea, vomiting, dyspepsia, stomatitis, abdominal pain, diarrhea, constipation, bad breath.
Immune system, skin and subcutaneous tissue disorders: hypersensitivity reactions, including anaphylactic and anaphylactoid reactions, anaphylactic shock, rash, urticaria, mucosal reactions, angioedema, fever, dyspnoea, pruritus; erythema, eczema, severe skin lesions: Stevens-Johnson syndrome and Lyell's syndrome.
General disorders: edema.
Very rare cases of bleeding have been reported with the use of acetylcysteine, most often associated with the development of hypersensitivity reactions. There have been cases of decreased platelet aggregation, but there is no clinical confirmation of this.
Expiration date
3 years.
Storage conditions
Store in the original packaging at a temperature not exceeding 25 °C.
Keep out of reach of children.
Packaging
10 tablets in a blister. 1 or 2 or 4 blisters in a cardboard pack.
Vacation category
Without a prescription.
Producer
Evertogen Life Sciences Limited.
Location of the manufacturer and its business address.
Plot No. S-8, S-9, S-13 and S-14, A.P.I.C, S.I.Z Pharma, Green Industrial Park, Polepally (BI), Yedcherla (EM), Mahabubnagar, IN - 509 301, India/
Plot No. S-8, S-9, S-13 & S-14, APIIC, Pharma Sez, Green Industrial Park, Polepally (V), Jadcherla (M), Mahabubnagar, In-509 301, India.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.