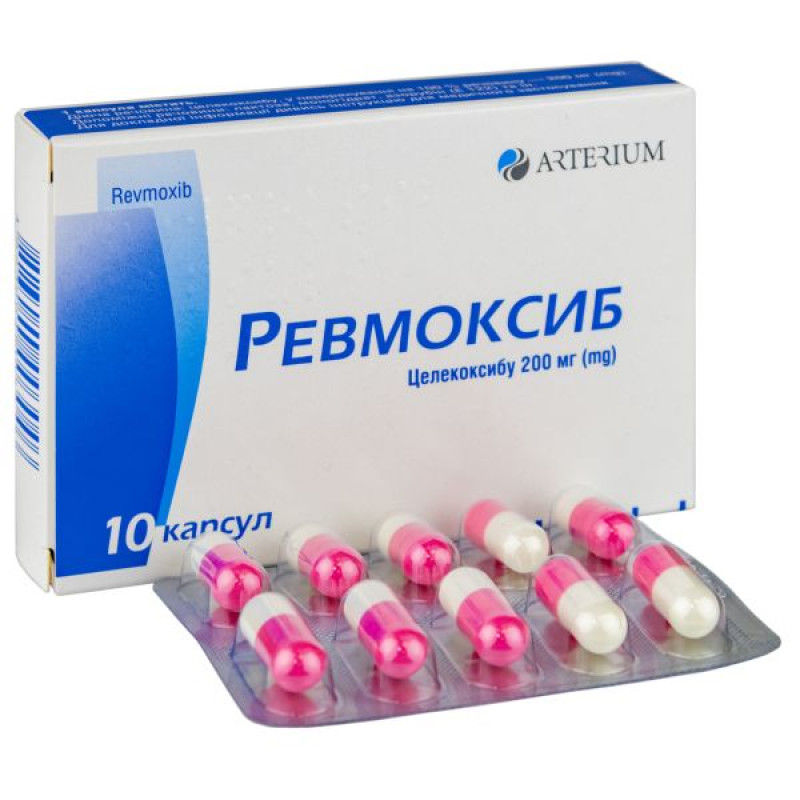
Revmoxib capsules 200 mg blister No. 10
Revmoxib capsules are indicated for:
for the relief of signs and symptoms of osteoarthritis, rheumatoid arthritis and ankylosing spondylitis; for the treatment of acute pain in adult patients; for the treatment of primary dysmenorrhea.Composition
Active ingredient: celecoxib;
1 capsule contains celecoxib in terms of 100% substance - 200 mg;
Excipients: lactose monohydrate (granulac-70); povidone; sodium lauryl sulfate; calcium stearate;
Capsule shell composition: gelatin, titanium dioxide (E 171), azorubine (E 122).
Contraindication
Hypersensitivity to celecoxib, aspirin or other nonsteroidal anti-inflammatory drugs. History of allergic-type reactions to sulfonamides. History of bronchial asthma, urticaria or other allergic-type reactions after the use of aspirin or other nonsteroidal anti-inflammatory drugs. Treatment of perioperative pain during coronary artery bypass grafting. Acute gastrointestinal bleeding.Method of application
Before deciding to use the drug, the potential benefits and risks of using the drug and other treatment options should be carefully weighed.
Each individual patient should be treated with the lowest effective dose for the shortest period of time consistent with the goals of treatment.
The drug can be taken regardless of meals.
Application features
Pregnant women
Celecoxib should be used during pregnancy only if the potential benefit outweighs the potential risk to the fetus.
Studies evaluating the effect of celecoxib on fetal ductus arteriosus closure have not been conducted. Therefore, it should not be used in women in the third trimester of pregnancy.
Children
Revmoxib, 200 mg capsules, is not indicated for use in children.
Drivers
If dizziness, vertigo, or drowsiness occur while taking celecoxib, you should refrain from driving or operating other machinery.
Overdose
No cases of overdose have been reported. It is known that doses up to 2400 mg/day for 10 days have not resulted in serious toxicity. Symptoms that occur after acute overdose of NSAIDs are usually limited to lethargy, drowsiness, nausea, vomiting and epigastric pain and are generally reversible with supportive treatment. Gastrointestinal bleeding may occur. Hypertension, acute renal failure, respiratory depression and, rarely, coma may occur. Anaphylactoid reactions have been reported with therapeutic use of NSAIDs, which can also occur with overdose.
In case of overdose with NSAIDs, patients should be treated symptomatically and supportively. There is no specific antidote. There is no information on the removal of celecoxib by hemodialysis, but despite its high degree of binding to plasma proteins (> 97%), dialysis is unlikely to be useful in cases of overdose. Within 4 hours after taking the drug, patients with symptoms of overdose or with a significant overdose can be induced to vomit and/or given activated charcoal (60-100 g for adults) and/or an osmotic laxative. Due to the high degree of binding of the drug to plasma proteins, forced diuresis, urine alkalization, hemodialysis or hemoperfusion may be ineffective.
Side effects
General: back pain, edema, accidental trauma, exacerbation of allergy, allergic reaction, chest pain, generalized edema, facial edema, fatigue, increased body temperature (hyperthermia), hot flashes, flu-like symptoms, pain (including peripheral), sepsis, sudden death, anaphylactoid reactions, angioedema, alveolar osteitis.
Interaction
Patients taking warfarin or similar drugs should be monitored for anticoagulant activity after starting celecoxib or making changes to their dosage, especially in the first few days, as these patients are at increased risk of developing bleeding complications.
Celecoxib can be used with low-dose aspirin. However, concomitant use of aspirin with celecoxib increases the incidence of gastrointestinal ulceration or other complications compared with use of celecoxib alone.
Revmoxib should not be used concomitantly with any doses of non-aspirin NSAIDs due to the potential increased risk of adverse reactions.
Storage conditions
Store in the original packaging at a temperature not exceeding 25 °C.
Keep out of reach of children.
Shelf life - 3 years.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.