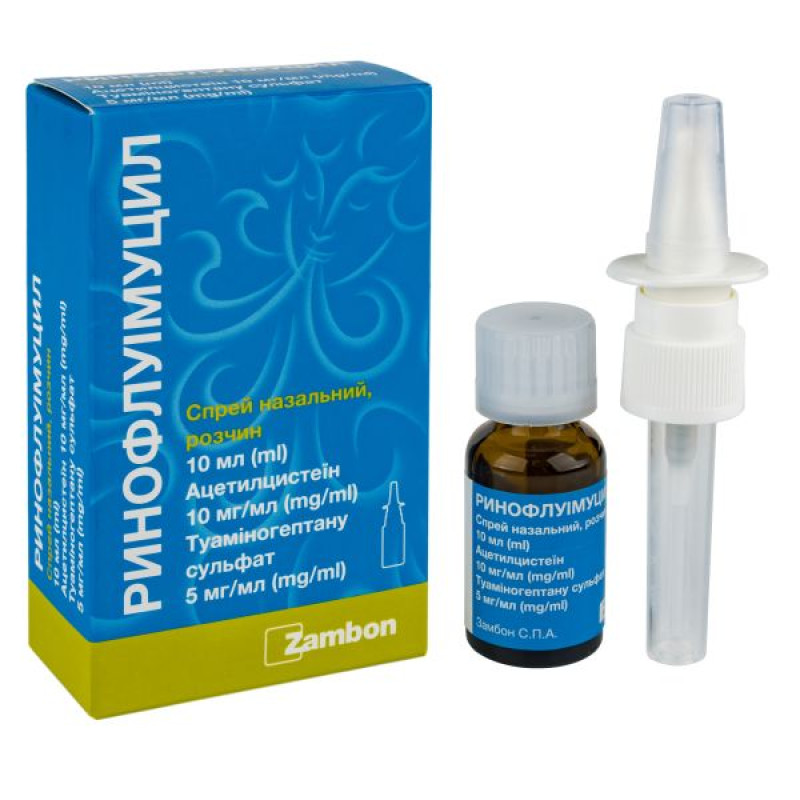
Rinofluimucil nasal spray bottle 10 ml

Instructions for Rinofluimucil nasal spray bottle 10 ml
Composition
active ingredients: 1 ml of the drug contains: acetylcysteine - 10 mg, tuaminoheptane sulfate - 5 mg; excipients: benzalkonium chloride, hypromellose, disodium edetate, sodium dihydrogen phosphate monohydrate, sodium hydrogen phosphate dodecahydrate, dithiothreitol, sorbitol (E 420), mint flavoring, ethanol 96%, sodium hydroxide, water for injections.
Dosage form
Nasal spray, solution.
Main physicochemical properties: practically transparent, colorless solution with a mint aroma.
Pharmacotherapeutic group
Remedies used for diseases of the nasal cavity.
ATX code. PBX R01A B08.
Pharmacological properties
Pharmacodynamics.
The drug has a mucolytic and anti-edematous effect, which is explained by the pharmacological properties of the active ingredients.
Acetylcysteine has a mucolytic effect due to the presence of a free sulfide group, which, by breaking the disulfide bonds of mucus glycoproteins, dilutes nasopharyngeal secretions.
Tuaminoheptane sulfate is a sympathomimetic amine that, when applied topically, exhibits a vasoconstrictor effect without systemic effects.
Pharmacokinetics.
The active components of the drug are not absorbed into the bloodstream in significant doses when administered systemically.
Indication
Acute and subacute rhinitis with thick purulent-mucous secretion, chronic rhinitis, vasomotor rhinitis, sinusitis.
Contraindication
Hypersensitivity to the active ingredient or to any other components of the drug;
pheochromocytoma;
angle-closure glaucoma;
hyperthyroidism;
children under 12 years old;
cardiovascular diseases, including hypertension;
concomitant use of other sympathomimetic agents, including other intranasal decongestants;
simultaneous use and use within 2 weeks after completion of treatment with monoamine oxidase inhibitors (MAOIs), including reversible MAOIs;
removal of the pituitary gland or previous operations with access to the dura mater.
Interaction with other medicinal products and other types of interactions
Despite the low degree of systemic absorption of tuaminoheptane when administered intranasally, the following potential interactions should be considered:
MAO inhibitors, including reversible ones, increase the risk of hypertensive crisis;
antihypertensive agents (including adrenergic neuron blockers and β-blockers) – will weaken the hypotensive effect;
cardiac glycosides – increase the risk of arrhythmias;
ergot alkaloids – increase the risk of ergotism;
antiparkinsonian drugs – increase the risk of cardiovascular toxicity;
Oxytocin – increases the risk of arterial hypertension.
Application features
The drug should be used with caution in patients suffering from occlusive vascular diseases, bronchial asthma, diabetes and taking β-blockers. Rinofluimucil should also be used with caution in children, taking into account that the drug is contraindicated for use in children under 12 years of age.
Long-term use of drugs containing vasoconstrictors can change the normal function of the mucous membrane of the nasal cavity and paranasal sinuses, thereby causing drug addiction. Therefore, frequent use over a long period of time may cause the development of side effects.
The drug should be used with caution in the elderly and in patients with prostatic hypertrophy due to the risk of developing urinary retention.
The use, especially for a long time, of local vasoconstrictor drugs may cause harmful effects; in this case, it is necessary to stop treatment and, if necessary, start appropriate therapy. In any case, in the absence of a full therapeutic effect within a few days, you should consult a doctor; in any case, the duration of treatment should not exceed 7 days.
At the doctor's discretion, the drug can be combined with appropriate antibacterial therapy.
Tuaminoheptane sulfate may give a positive result in a doping test.
This drug is not intended for ophthalmic use.
Rinofluimucil contains benzalkonium chloride, which has an irritating effect and can cause skin reactions or bronchospasm.
Do not allow the medicine to get into your eyes.
Use during pregnancy or breastfeeding
Pregnancy
Separate data on the use of acetylcysteine during pregnancy do not indicate an adverse effect of this drug on the course of pregnancy, the health of the fetus or newborn. Significant epidemiological data are currently lacking. Animal studies do not indicate a direct or indirect harmful effect of acetylcysteine on reproductive function.
There are no data on the effects of tuaminoheptane during pregnancy or data from similar studies in animals.
The use of the drug during pregnancy is not recommended.
Breast-feeding
There is no data on the excretion of acetylcysteine or tuaminoheptane into breast milk, therefore this drug should not be used during breastfeeding.
The ability to influence the reaction speed when driving or working with other mechanisms
There is no data indicating the effect of the drug on concentration and changes in reaction speed.
Method of administration and doses
The drug is administered into the nasal cavity using a special spray.
Adults - 2 doses of spray (2 presses on the valve) in each nostril 3-4 times a day.
Children over 12 years old - 1 dose of spray (1 press on the valve) in each nostril 3-4 times
per day.
Do not exceed recommended doses.
After first opening, the product can be used within 20 days.
Before administering the spray, you must:
Remove the cap from the solution bottle.
Remove the protective cap from the sprayer.
Attach the sprayer to the bottle.
Remove the cap from the sprayer.
Activate the sprayer by pressing again.
Children.
The drug is used by children aged 12 years and older.
Overdose
Symptoms: possible development of arterial hypertension, photophobia, severe headache, feeling of tightness in the chest, while in children possible development of hypothermia with pronounced depression of consciousness.
Such reactions require immediate adequate medical treatment.
Treatment: symptomatic.
Adverse reactions
Frequent use of the drug in high doses may cause the development of sympathomimetic side effects (increased excitability, rapid heartbeat, tremor, etc.). Sometimes dryness of the nasal and throat mucosa, as well as inflammation of the sebaceous glands may occur. Such effects completely disappear after discontinuation of treatment.
The listed adverse reactions may be associated with the use of Rinofluimucil; the frequency of their occurrence is unknown (cannot be estimated based on the available data).
On the part of the immune system: hypersensitivity.
Mental disorders: (especially with prolonged and/or excessive use) anxiety, hallucinations, delusions.
Nervous system: (especially with prolonged and/or excessive use) headache, anxiety, agitation, insomnia, tremor.
Cardiovascular system: (especially with prolonged and/or excessive use) palpitations, tachycardia, arrhythmias, arterial hypertension.
On the part of the respiratory system: (especially with prolonged and/or excessive use) dryness in the nasal cavity and throat, discomfort in the nasal cavity, nasal congestion.
Gastrointestinal: nausea.
Skin and subcutaneous tissue disorders: urticaria, skin rash.
From the urinary system: urinary retention.
Systemic disorders and complications at the injection site: (especially with prolonged and/or excessive use) irritability, tolerance to the drug.
Expiration date
2.5 years.
Do not use after the expiration date.
After first opening, the medicine should be stored for no more than 20 days.
Conditions and shelf life
Store at a temperature not exceeding 25°C.
Packaging
10 ml in a bottle; 1 bottle with a sprayer in a cardboard box.
Vacation category
Without a prescription.
Producer
Zambon SPA/Zambon SPA
Location of the manufacturer and address of its place of business
Via della Chimica, 9 - 36100 Vicenza (province of Vicenza), Italy/Via della Chimica, 9 - 36100 Vicenza (VI), Italy.
Applicant
Zambon S.P.A. / Zambon SPA
Location of the applicant and its address of place of business
Via Lillo del Duca, 10 - 20091 Bresso, Milan, Italy/Via Lillo del Duca, 10 - 20091 Bresso, Milan, Italy.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.