Seretide Diskus 50mcg/100mcg 60 doses

Inhalation powder, metered dose. "Seretide™ Diskus™" is indicated for use in:
bronchial asthma. Regular treatment of bronchial asthma in patients who are indicated for combination therapy with a long-acting β2-adrenomimetic and an inhaled corticosteroid. bronchial obstructive pulmonary disease. Maintenance therapy of chronic obstructive pulmonary disease (COPD) in patients with FEV1 < 60% of predicted values (before bronchodilator inhalation) and a history of repeated exacerbations, in whom severe symptoms of the disease persist despite regular bronchodilator therapy.Composition
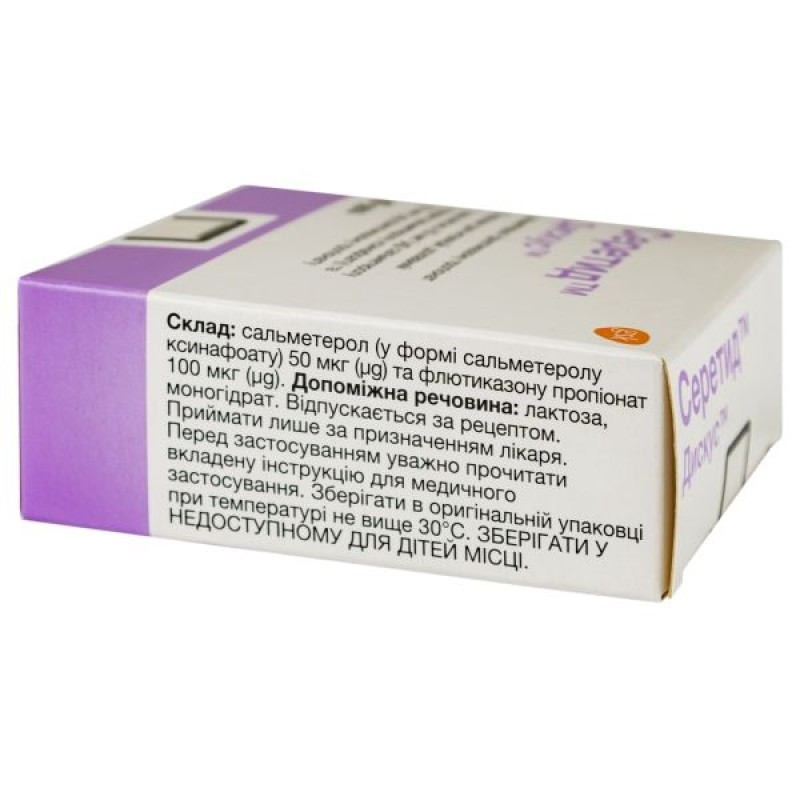
1 dose of the drug contains 50 mcg of salmeterol (in the form of salmeterol xinafoate) micronized and 100 mcg of fluticasone propionate (micronized);
excipient: lactose monohydrate.
Contraindication
Hypersensitivity to any of the components of the drug.
Method of application
Seretide Diskus is for inhalation use only.
Recommended doses.
Asthma.
Adults and children aged 12 years and over:
- one inhalation (50 mcg salmeterol/100 mcg fluticasone propionate) twice daily.
For the treatment of adults and adolescents with moderate persistent asthma, Seretide Diskus can be used as initial maintenance therapy when rapid control of symptoms is required. In such cases, the recommended starting dose is one inhalation of 50 micrograms of salmeterol and 100 micrograms of fluticasone propionate twice daily.
Children aged 4 to 12 years: one inhalation (50 mcg salmeterol/100 mcg fluticasone propionate) twice daily.
The maximum daily dose of fluticasone propionate in Seretide Diskus for the treatment of children is 100 mcg twice a day.
Chronic obstructive pulmonary disease.
Adults: - one inhalation (50 mcg salmeterol/500 mcg fluticasone propionate) twice daily.
Application features
Pregnant women
The use of Seretide Diskus during pregnancy is appropriate only in cases where the expected benefit to the mother outweighs any risk to the fetus.
The use of Seretide Diskus during breastfeeding is appropriate only if the expected benefit to the mother outweighs any possible risk to the child.
Children
There are no data on the use of Seretide Diskus in children under 4 years of age, therefore its use is not recommended.
Drivers
Studies of the effect of Seretide Diskus on this activity have not been conducted, but given the pharmacology of both drugs, it can be assumed that there is no effect.
Overdose
According to clinical trials, there is no information on overdose with Seretide Diskus.
Side effects
Infections and infestations: candidiasis of the mouth and throat, esophageal candidiasis, pneumonia, bronchitis.
Immune system disorders: hypersensitivity skin reactions, edema, shortness of breath, bronchospasm, anaphylactic reactions, including anaphylactic shock.
Mental disorders: anxiety, sleep disorders, hyperactivity and agitation, depression, aggression.
Nervous system disorders: headache, tremor.
Visual disorders: cataracts, glaucoma, impaired visual acuity.
Cardiac disorders: palpitations, tachycardia, cardiac arrhythmia.
Respiratory disorders: nasopharyngitis, throat irritation, hoarseness/dysphonia, sinusitis, paradoxical bronchospasm.
Storage conditions
Store in the original packaging at a temperature not exceeding 30 ° C. Keep out of the reach of children.
Shelf life - 2 years.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.