Smecta oral suspension 3 g sachet 10.27 g No. 12
Instructions Smecta oral suspension 3 g sachet 10.27 g No. 12
Composition
active ingredient: diosmectite;
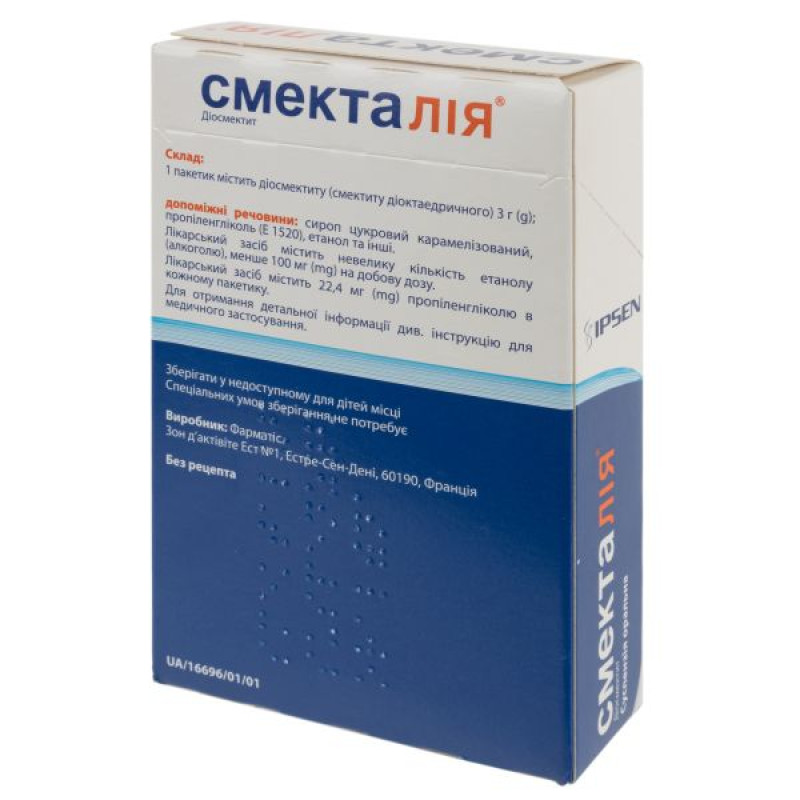
1 sachet contains diosmectite (dioctahedral smectite) 3 g;
excipients: xanthan gum; citric acid, monohydrate; ascorbic acid; potassium sorbate; sucralose; caramel-cocoa flavoring (mixture of natural and synthetic flavors, sugar color (E 150d), caramelized sugar syrup, propylene glycol (E 1520), water, ethanol, caffeine); purified water.
Dosage form
Oral suspension.
Main physicochemical properties: homogeneous suspension from beige to light beige color with a characteristic smell of caramel.
Pharmacotherapeutic group
Antidiarrheal drugs; drugs used to treat infectious and inflammatory diseases of the intestines. Various enterosorbents.
ATX code A07B C05.
Pharmacological properties
Pharmacodynamics
Diosmectite is a double silicate of aluminum and magnesium.
Due to its stereometric structure and high plastic viscosity, diosmectite has a high enveloping ability for the mucous membrane of the digestive tract. Diosmectite, by interacting with glycoproteins of the mucous membrane, increases the resistance of mucus to irritants. Diosmectite, affecting the barrier function of the mucous membrane of the digestive system and due to its high ability to bind to the mucous membrane, protects the mucous membrane of the digestive tract. Diosmectite is radiolucent, does not stain feces and in normal doses does not affect the physiological time of passage through the intestine.
Pharmacokinetics
Due to the structure of diosmectite, Smecta is not adsorbed or metabolized.
Indication
Short-term treatment of acute diarrhea in adults and children aged 15 years and older, as an adjunct to diet.
Contraindication
Hypersensitivity to diosmectite or to one of the excipients.
Intestinal obstruction.
Interaction with other medicinal products and other types of interactions
The adsorbing properties of this medicinal product may affect the extent and/or rate of absorption of other substances, therefore it is recommended not to use other medicinal products simultaneously with Smecta.
Application features
Diosmectite should be taken with caution in patients with a history of severe chronic constipation.
Treatment does not exclude rehydration if necessary.
The volume of rehydration using oral rehydration solution or intravenous rehydration depends on the intensity of diarrhea, the patient's age, and the characteristics of the course of the disease.
The patient should be informed about the need to:
rehydration using a significant volume of salty or sweet liquids to compensate for fluid loss caused by diarrhea (the average daily water requirement of an adult is 2 liters);
Maintaining food intake while diarrhea persists:
– excluding certain foods, especially raw vegetables and fruits, green vegetables, spicy dishes, as well as frozen foods or drinks;
– with preference for baked meat and rice.
This medicine contains a small amount of ethanol (alcohol), less than 100 mg/dose.
If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicine.
Use during pregnancy or breastfeeding
There are no reliable data on teratogenesis in animals.
To date, no specific fetal malformations or fetotoxic effects have been observed in clinical settings. However, studies of the effects of Smectaly on pregnant women sufficient to exclude any risk have not been conducted.
Therefore, given that Smecta is not absorbed, the use of this medicine during pregnancy is only recommended if necessary.
Ability to influence reaction speed when driving vehicles or other mechanisms
No studies have been conducted on the effects of this medicinal product on the ability to drive and use machines. However, it is expected to have little or no effect.
Method of administration and doses
For oral use in adults and children aged 15 years and over.
Dosage
1 sachet (diosmectite, 3 g) followed by 1 more sachet after each loose stool; however, no more than 6 sachets per day.
The maximum duration of treatment is 3 days.
Method of application
It is recommended to knead the contents of the sachet with your fingers before opening it, bringing it to a liquid state. The contents of the sachet can be swallowed undiluted or mixed with a small amount of water and drunk.
It is advisable to take the medicine separately from food.
Children.
Use for the treatment of children aged 15 years and over.
Overdose
Overdose can lead to increased side effects, constipation.
Adverse reactions
The frequency of adverse reactions is classified as follows: common (≥1/100 to ≤1/10); uncommon (≥1/100 to ≤1/100).
Gastrointestinal tract
Common: constipation, which usually resolves after dose reduction, but in some cases may require discontinuation of treatment.
During the post-marketing period, hypersensitivity reactions (frequency unknown) including urticaria, rash, pruritus, and angioedema have been reported with diosmectite.
Constipation has also been reported.
Expiration date
3 years.
Storage conditions
Does not require special storage conditions.
Keep out of reach of children.
Packaging
10.27 g of oral suspension in a sachet; 12 sachets in a cardboard box.
Vacation category
Without a prescription.
Producer
Pharmatis – Estrees Saint Denis/Pharmatis – Estrees Saint Denis.
Beaufour Ipsen Industrie – Dreux.
Location of the manufacturer and its business address
Pharmatis – Estrees Saint Denis/Pharmatis – Estrees Saint Denis.
Zone d'Activites Est n01, Estrees Saint Denis, 60190, France.
Beaufour Ipsen Industrie – Dreux.
Rue Ethe Virton, Dreux, 28100, France.
Applicant
IPSEN PHARMA/IPSEN PHARMA.
Applicant's location
65, quai Georges Gorse-92100 Boulogne Billancourt, France/65, quai Georges Gorse-92100 Boulogne Billancourt, France.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.