Soda-Buffer solution for infusions 4.2% bottle 100 ml
Instructions for Soda-Buffer solution for infusions 4.2% bottle 100 ml
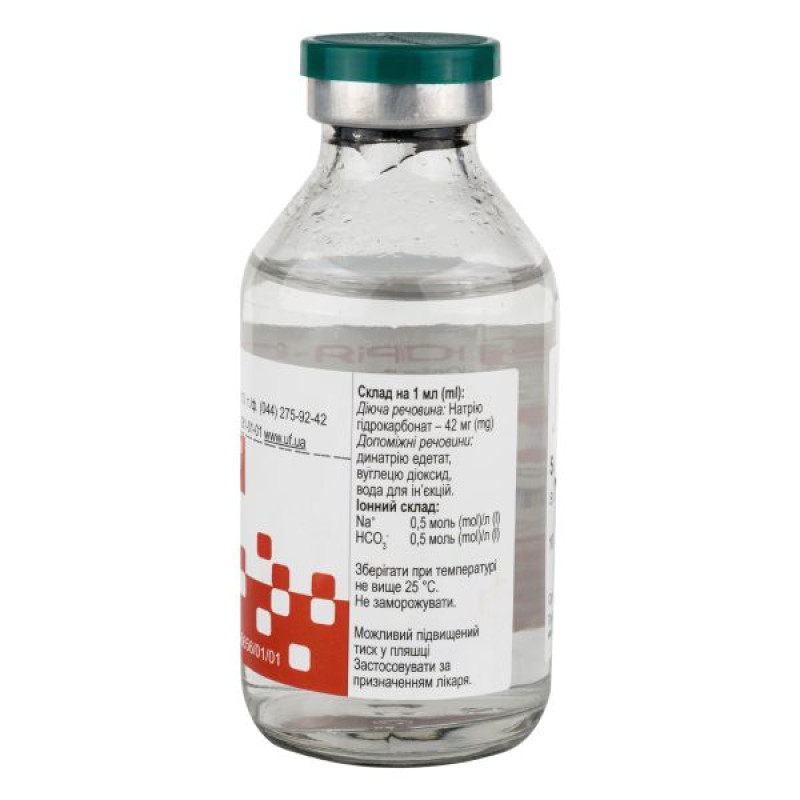
Composition
active ingredient: sodium bicarbonate;
1 ml of solution contains 42 mg of sodium bicarbonate;
Excipients: disodium edetate, carbon dioxide, water for injections.
Dosage form
Solution for infusion.
Main physicochemical properties: clear, colorless liquid.
Ionic composition per 1 liter of the drug: sodium ion – 0.5 mol; bicarbonate ion – 0.5 mol. Theoretical osmolarity – 1000 mOsm/l. pH 7.0-8.5.
Pharmacotherapeutic group
Intravenous solutions. Sodium bicarbonate. ATX code B05X A02.
Pharmacological properties
Pharmacodynamics
A remedy for restoring the alkaline state of the blood and correcting metabolic acidosis. When sodium bicarbonate is dissociated, bicarbonate anion is released, it binds hydrogen ions to form carbonic acid, which then decomposes into water and carbon dioxide, which is released during breathing. The solution, adjusted to a pH of 7.3-7.8, prevents abrupt alkalization and provides a smooth correction of acidosis while simultaneously increasing the alkaline reserves of the blood. The drug also increases the excretion of sodium and chlorine ions from the body, increases osmotic diuresis, alkalizes urine, and prevents the precipitation of uric acid in the urinary system. Bicarbonate anion does not penetrate into cells.
Pharmacokinetics
Not researched.
Indication
Uncompensated metabolic acidosis in intoxications of various etiologies, severe postoperative period, extensive and/or deep burns, shock, diabetic coma, prolonged diarrhea, uncontrollable vomiting, acute massive blood loss, severe liver and kidney damage, prolonged febrile states, severe hypoxia of newborns. An absolute indication is a decrease in blood pH below 7.2.
Contraindication
Metabolic or respiratory alkalosis, hypokalemia, hypernatremia.
Interaction with other medicinal products and other types of interactions
May enhance the antihypertensive effect of reserpine.
Application features
It is necessary to monitor the pH and acid-base status of the blood. Patients with concomitant heart or kidney diseases may develop heart failure and edema. In case of too rapid equilibration of acidosis, in particular in case of impaired pulmonary ventilation, the rapid release of CO2 may increase cerebral acidosis. Small amounts of Soda-buffer®, administered together with other infusion solutions that have an acidic pH, serve as a neutralizing agent and prevent the occurrence of post-infusion phlebitis (glucose solutions of various concentrations, Ringer's solution, sodium chloride solution, ciprofloxacin and some other fluoroquinolones).
Use during pregnancy or breastfeeding
It is not known whether sodium bicarbonate causes fetal harm when administered to pregnant women or whether it can affect reproductive function. Therefore, the drug should be administered to pregnant women only if the expected benefit to the mother outweighs the potential risk to the fetus.
The use of the drug during breastfeeding is possible only if absolutely necessary.
The ability to influence the reaction speed when driving or working with other mechanisms
Data are missing due to the use of the drug exclusively in a hospital setting.
Method of administration and doses
Administer to adults and children intravenously by drip at a rate of 1.5 mmol/kg per hour (3 ml of 4.2% Soda-buffer®/kg per hour).
Newborns should be administered intravenously at a dose of 1-2 ml per 1 kg of body weight at a time under the control of acid-base and water-electrolyte balance indicators.
In the case of correction of metabolic acidosis, the dosage is determined taking into account the level of acid-base imbalance. The dose is calculated depending on the blood gas parameters using the formula:
Volume of 0.5 molar buffered sodium bicarbonate 4.2% in ml =
base deficiency (-BE) x kg of patient's body weight x 0.3 x 2
(the factor 0.3 corresponds to the proportion of extracellular fluid compared to total fluid).
The maximum dose of the drug for adults is 300 ml (400 ml with increased body weight) per day, for children - from 100 to 200 ml depending on body weight.
Children
Can be used on children from birth.
Overdose
Due to the buffering properties of the drug, the likelihood of developing alkalosis in case of overdose is significantly reduced. If the dose of the drug is exceeded, the development of alkalosis, hypernatremia and hyperosmia, tetanic convulsions is possible. If clinical manifestations of alkalosis appear (convulsions, including with manifestations of tetany, agitation, decreased potassium and calcium levels and increased sodium levels in the blood, increased pH), stop administering the drug, if necessary, administer isotonic sodium chloride solution or 5% glucose solution. If there is a risk of developing tetany, administer 1-3 g of calcium gluconate intravenously to adults.
Side effects
Nausea, vomiting, anorexia, stomach pain, headache, anxiety. Arterial hypertension.
Expiration date
2 years.
Storage conditions
Store out of reach of children at a temperature not exceeding
Incompatibility
Acidic substances (ascorbic, nicotinic and other acids), alkaloids (atropine, apomorphine, caffeine, theobromine, papaverine), cardiac glycosides, salts of calcium, magnesium, heavy metals (iron, copper, zinc) cannot be dissolved in Soda-buffer® solution, as precipitation or hydrolysis of organic compounds occurs. Do not mix with phosphate-containing solutions.
Packaging
20 ml, 100 ml and 200 ml in glass bottles.
Vacation category
According to the recipe.
Producer
LLC "Yuria-Pharm"
Address
Ukraine, 18030, Cherkasy region, Cherkasy city, Kobzarska st., 108. Tel.: (044) 281-01-01.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.