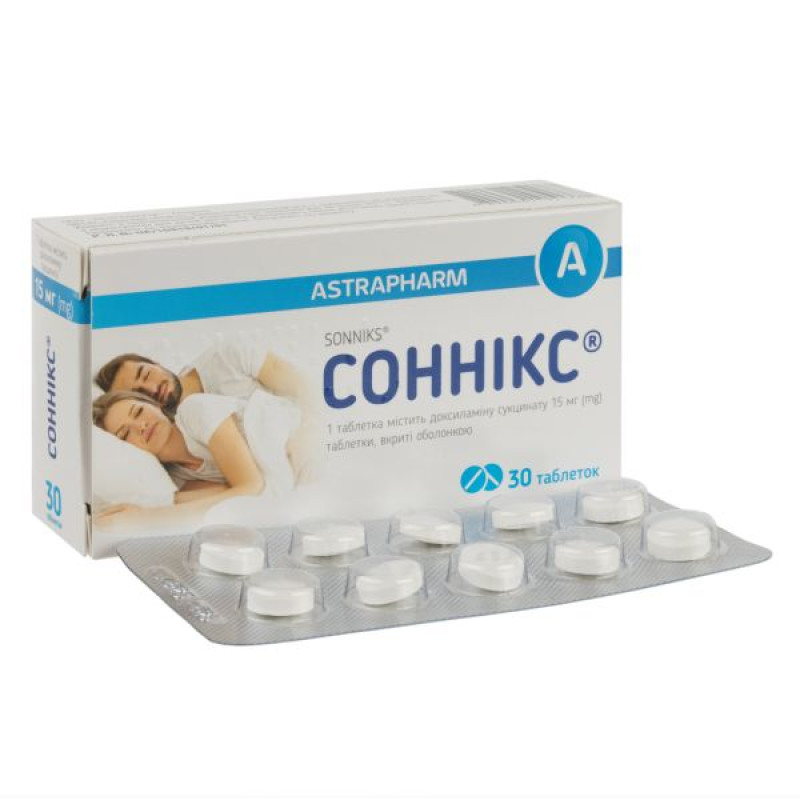
Sonnix film-coated tablets 15 mg No. 30
Instructions for Sonnix film-coated tablets 15 mg No. 30
Composition
active ingredient: doxylamine;
1 tablet contains doxylamine succinate 15 mg;
excipients: lactose monohydrate; croscarmellose sodium; microcrystalline cellulose; magnesium stearate; Cele CoatTM coating (hypromellose, polyethylene glycol (macrogol) 6000, titanium dioxide (E171)).
Dosage form
Film-coated tablets.
Main physicochemical properties: round tablets with a biconvex surface, covered with a white shell, two layers are visible on the break.
Pharmacotherapeutic group
Antihistamines for systemic use. ATX code R06A A09.
Hypnotics and sedatives. ATX code N05C M.
Pharmacological properties
Pharmacodynamics.
Sonnix® is a hypnotic drug of the ethanolamine class from the group of histamine H1-receptor blockers, which has a sedative and m-cholinoblocking effect. It reduces the time to fall asleep, increases the duration and quality of sleep, without changing the phases of sleep.
Pharmacokinetics.
Doxylamine succinate is well absorbed from the gastrointestinal tract. The maximum concentration in the blood plasma is reached 2 hours after taking the tablets. The average half-life from the blood plasma is on average 10 hours.
Doxylamine succinate undergoes biotransformation in the liver. Doxylamine succinate is partially metabolized in the liver by demethylation and N-acetylation. The half-life may be significantly increased in elderly patients or in patients with renal or hepatic insufficiency. The various metabolites formed during the breakdown of the molecule are not quantitatively significant, since 60% of the administered dose is found in the urine as unchanged doxylamine.
There are no data on the ability of doxylamine succinate to penetrate into breast milk, but this possibility cannot be ruled out.
Indication
Periodic and transient insomnia.
Contraindication
Hypersensitivity to the components of the drug; angle-closure glaucoma in the patient's history or in the family history; difficulty urinating (urethra and prostate disease).
Interaction with other medicinal products and other types of interactions
Alcohol enhances the sedative effect of most H1 antihistamines. Alcoholic beverages and medications containing ethanol should be avoided.
The following combinations of Sonnix® should be considered with:
– atropine and atropine-like drugs (imipramine antidepressants, anticholinergic antiparkinsonian drugs, atropine antispasmodics, disopyramide, phenothiazine neuroleptics) due to the occurrence of such side effects as urinary retention, constipation, dry mouth;
– other antidepressants that affect the central nervous system (morphine derivatives (painkillers; drugs used to treat cough and substitution therapy), neuroleptics; barbiturates, benzodiazepines; anxiolytics other than benzodiazepines; sedative antidepressants (amitriptyline, doxepin, mianserin, mirtazapine, trimipramine); sedative H1-antihistamines; centrally acting antihypertensives; others: baclofen, pizotifen, thalidomide) due to increased depression of the central nervous system.
Application features
Since the drug contains lactose, it is contraindicated in cases of congenital galactosemia, glucose-galactose malabsorption syndrome, and lactase deficiency.
Like all hypnotics or sedatives, doxylamine succinate may exacerbate sleep apnea syndrome (increased number and duration of breathing stops).
H1-antihistamines should be used with caution in elderly patients due to the risk of dizziness, which may increase the risk of falls (for example, when people get up at night) with consequences that are often serious in this category of patients.
To prevent drowsiness during the day, it is necessary to remember that the duration of sleep after using the drug should be at least 7 hours.
Alcohol should be avoided while using the drug.
Use during pregnancy or breastfeeding
The drug is contraindicated during pregnancy or breastfeeding.
Ability to influence reaction speed when driving vehicles or other mechanisms
Sonnix® affects the speed of psychomotor reactions, so you should refrain from driving or using other mechanisms.
Method of administration and doses
For oral use. Take 15-30 minutes before bedtime.
The recommended dose is 7.5-15 mg per day (½-1 tablet per day). If necessary, the dose can be increased to 30 mg per day (2 tablets per day).
A dose reduction is recommended for elderly patients and patients with renal or hepatic insufficiency.
The duration of the treatment course is 2-5 days.
If insomnia persists for longer than 5 days, you should consult a doctor regarding the appropriateness of further use of the drug.
Children.
The efficacy and safety of Sonnix® in children under 15 years of age have not been established, therefore the drug should not be prescribed to this age group of patients.
Overdose
Symptoms: The first signs of acute poisoning are drowsiness and signs of anticholinergic effects: agitation, dilated pupils, paralysis of accommodation, dry mouth, flushing of the face and neck, hyperthermia, sinus tachycardia. Delirium, hallucinations and athetoid movements are more common in children; they are sometimes precursors of convulsions - rare complications of severe poisoning. Even if convulsions do not occur, acute doxylamine poisoning sometimes causes rhabdomyolysis, which can be complicated by acute renal failure. This muscle disorder is common, requiring systematic screening by measuring creatine phosphokinase activity.
Treatment: administration of activated charcoal (50 g for adults and 1 g/kg for children), if necessary, symptomatic treatment. Anticonvulsants and artificial ventilation are prescribed as indicated.
Adverse reactions
In the morning after taking the drug in the evening, slowed reactions and dizziness may occur, so to prevent falling, sudden movements should be avoided. Rarely, anticholinergic effects develop: constipation, dry mouth, accommodation disorders, palpitations, urinary retention.
Daytime drowsiness: if this effect develops, the dose should be reduced.
Allergic reactions are possible, including skin rashes and itching.
Expiration date
3 years.
Storage conditions
Store in the original packaging at a temperature not exceeding 25 °C.
Keep out of reach of children.
Packaging
10 tablets in a blister; 3 blisters in a box.
Vacation category
No. 10 - Without a prescription.
#30 - By prescription.
Producer
"ASTRAPHARM" LLC.
Location of the manufacturer and address of its place of business
08132, Ukraine, Kyiv region, Kyiv-Svyatoshynskyi district, Vyshneve, Kyivska st., 6.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.