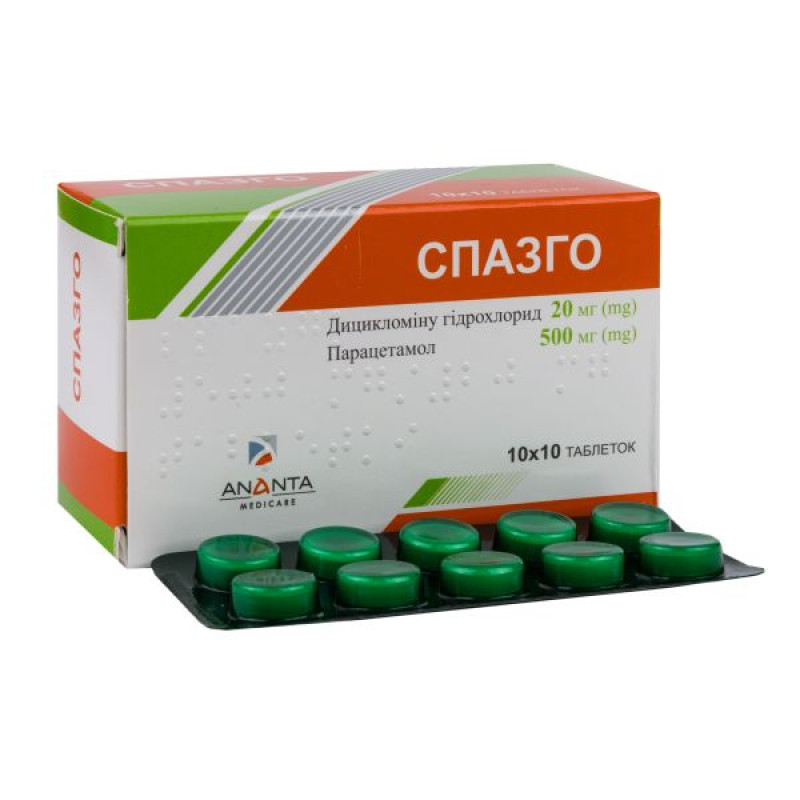
Spazgo tablets No. 100
Instructions for Spazgo tablets No. 100
Composition
active ingredients: paracetamol, dicyclomine hydrochloride;
1 tablet contains paracetamol 500 mg and dicyclomine hydrochloride 20 mg;
excipients: corn starch, gelatin, sodium methylparaben (E 219), sodium propylparaben (E 217), sodium metabisulfite (E 223), polyvinylpyrrolidone (K-30), magnesium stearate, talc, sodium starch glycolate.
Dosage form
Pills.
Main physicochemical properties: tablets are round, flat, white or almost white in color, with a score on one side, with a bevel.
Pharmacotherapeutic group
Analgesics and antipyretics. Paracetamol, combinations without psycholeptics. ATX code N02B E51.
Pharmacological properties
Pharmacodynamics
Paracetamol acts as an analgesic and antipyretic. The analgesic and antipyretic effects of paracetamol (a non-opiate, non-salicylate analgesic) are associated with the drug's effect on the thermoregulatory center in the hypothalamus and its ability to inhibit prostaglandin synthesis.
Dicyclomine hydrochloride is a tertiary amine. It has anticholinergic activity and reduces the tone of smooth muscles, eliminates pain, blocks antagonistic activity. Dicyclomine hydrochloride selectively paralyzes M-cholinergic structures, blocking the transmission of impulses from postganglionic cholinergic nerves to the effector organs innervated by them. Causes relaxation of smooth muscles, exhibiting an antispasmodic effect in spasms of smooth muscles of the stomach, intestines, biliary tract, urogenital and vascular systems.
Indication
Pain syndromes with a spastic component of various origins:
headache; toothache; muscle pain, neuralgia; rheumatic pain, radiculitis; renal colic; menstrual pain.
Contraindication
Glaucoma, tachycardia, urinary tract obstruction, myasthenia gravis, hypersensitivity to the components of the drug, severe renal and/or hepatic dysfunction, glucose-6-phosphate dehydrogenase deficiency, alcoholism, blood diseases, including anemia, leukopenia. Obstructive diseases of the digestive tract, urogenital tract, biliary tract. Peptic ulcer of the stomach or duodenum. Reflux esophagitis. Acute bleeding. Benign prostatic hypertrophy with a tendency to urinary retention. Dynamic intestinal obstruction. Severe liver and kidney diseases. Congenital hyperbilirubinemia (Gilbert, Dubin-Johnson and Rotor syndromes).
Interaction with other medicinal products and other types of interactions
The interaction features of the drug are due to the properties of its components.
Paracetamol, which is part of the drug, reduces the effectiveness of diuretics, and also increases the risk of hepatotoxic reactions when taken together with barbiturates, diphenyl, carbamazepine, rifampicin and other inducers of microsomal liver enzymes, as well as anticonvulsants. The rate of absorption of paracetamol may increase when used together with metoclopramide and domperidone and decrease when used together with cholestyramine. The effect of paracetamol is enhanced when combined with codeine, ascorbic acid, scopolamine, chlorphenamine, propyphenazone and caffeine. Simultaneous use of paracetamol with azidothymidine can lead to the development of neutropenia. The anticoagulant effect of warfarin and other coumarins is enhanced with prolonged regular use of paracetamol. The risk of bleeding increases. Periodic administration does not matter. The simultaneous use of paracetamol with nonsteroidal anti-inflammatory drugs increases the risk of kidney complications. The simultaneous use of paracetamol with hepatotoxic drugs increases the toxic effect of the drugs on the liver.
The effect of dicyclomine hydrochloride is enhanced by amantadine, antipsychotic agents, benzodiazepines, MAO inhibitors, narcotic analgesics, nitrates and nitrites, sympathomimetics, tricyclic antidepressants, anticholinergics, corticosteroids; reduced by antacids. Dicyclomine hydrochloride enhances the effect of digoxin.
Application features
It is necessary to consult a doctor regarding the possibility of using the drug in patients with impaired kidney and liver function.
Before using the drug, it is necessary to consult a doctor if the patient is using warfarin or similar drugs that have an anticoagulant effect.
When using paracetamol, it is necessary to monitor the peripheral blood picture and liver function. It is not recommended to use it simultaneously with other drugs containing paracetamol due to the possibility of overdose.
When prescribing the drug for a period of more than 3 days, a doctor's monitoring of the patient's condition is required.
It should be used with caution in elderly patients and people who abuse alcohol.
Use with caution in heart failure, pyloric stenosis, and impaired renal and hepatic function.
May aggravate gastroesophageal reflux.
The risk of hepatotoxic effects of paracetamol is increased in patients with alcoholic liver damage.
The drug should be prescribed with caution to patients with arterial hypotension, a tendency to bronchospasm, as well as with increased individual sensitivity to nonsteroidal anti-inflammatory drugs. With prolonged use, the cellular composition of peripheral blood and the state of kidney function should be monitored.
Dicyclomine should be prescribed with caution in nonspecific ulcerative colitis (risk of paralytic obstruction), hiatal hernia accompanied by reflux esophagitis.
Patients taking anticholinergic drugs may experience psychosis, confusion, ataxia, coma, euphoria, weakness, insomnia, agitation, and inappropriate emotional displays (symptoms subside within 12-24 hours after dose reduction).
Use with caution at high ambient temperatures (due to decreased sweating, the likelihood of hyperthermia and heat stroke increases).
Patients who take analgesics every day for mild arthritis should consult a doctor.
Do not exceed the indicated doses.
If symptoms persist, you should consult a doctor.
If the headache becomes persistent, you should see a doctor.
Ability to influence reaction speed when driving vehicles or other mechanisms
Considering that the drug may reduce the speed of psychomotor reactions in sensitive patients, it is better to refrain from driving vehicles and working with other mechanisms that require concentration of attention during the period of therapy with the drug.
Use during pregnancy or breastfeeding
The drug should not be used by women during pregnancy or breastfeeding.
Method of administration and doses
The drug should be taken orally, washed down with a small amount of liquid (200 ml).
Adults and children over 15 years of age: 1 tablet, depending on the severity of the pain, 1 to 4 times a day.
Children aged 7 to 13 years: ½ tablet 1 to 2 times a day;
Children aged 13 to 15 years: 1 tablet 1 to 3 times a day.
The maximum daily dose for adults is 2 tablets 4 times a day.
The duration of treatment is determined by the doctor individually, depending on the patient's condition and reaction.
Children
The drug should not be prescribed to children under 7 years of age.
Overdose
Symptoms of overdose caused by paracetamol. Liver damage is possible in adults who have taken 10 g or more of paracetamol, and in children who have taken more than 150 mg/kg of body weight. In patients with risk factors (long-term treatment with carbamazepine, phenobarbitone, phenytoin, primidone, rifampicin, St. John's wort or other drugs that induce liver enzymes; regular use of excessive amounts of ethanol; glutathione cachexia (digestive disorders, cystic fibrosis, HIV infection, starvation, cachexia) the use of 5 g or more of paracetamol can lead to liver damage.
Symptoms of overdose in the first 24 hours: pallor, nausea, vomiting, anorexia and abdominal pain. Liver damage may become apparent 12-48 hours after overdose. Glucose metabolism disorders and metabolic acidosis may occur. In severe poisoning, liver failure may progress to encephalopathy, hemorrhage, hypoglycemia, coma and death. Acute renal failure with acute tubular necrosis may present with severe lumbar pain, hematuria, proteinuria and may develop even in the absence of severe liver damage. Cardiac arrhythmia and pancreatitis have also been reported.
With prolonged use of the drug in high doses, aplastic anemia, pancytopenia, agranulocytosis, neutropenia, leukopenia, thrombocytopenia may develop from the hematopoietic system. When taking high doses, from the central nervous system - dizziness, psychomotor agitation and disorientation; from the urinary system - nephrotoxicity (renal colic, interstitial nephritis, capillary necrosis).
In case of overdose, urgent medical attention is required. The patient should be taken to hospital immediately, even if there are no early symptoms of overdose. Symptoms may be limited to nausea and vomiting or may not reflect the severity of the overdose or the risk of organ damage. Treatment with activated charcoal should be considered if the overdose of paracetamol has been taken within 1 hour. The concentration of paracetamol in the blood plasma should be measured 4 hours or later after ingestion (earlier concentrations are unreliable). Treatment with N-acetylcysteine can be used within 24 hours of paracetamol ingestion, but the maximum protective effect occurs when it is used within 8 hours of ingestion. The effectiveness of the antidote decreases sharply after this time. If necessary, the patient should be given N-acetylcysteine intravenously according to the established dose list. In the absence of vomiting, oral methionine can be used as a suitable alternative in remote areas outside the hospital.
Overdose is characterized by a two-phase nature: first, central nervous system excitation occurs, manifested by restlessness, the appearance of illusions, hallucinations, persistent mydriasis, tachycardia, and arterial hypertension. Then, central nervous system depression occurs, up to a comatose state.
In the first 24 hours - pale skin, nausea, anorexia, vomiting and abdominal pain, after 12-48 hours - kidney and liver damage with the development of liver failure (increased activity of hepatic transaminases, dehydrogenase, increased concentration of bilirubin, prothrombin); tachycardia, arrhythmias; change in respiratory rate; pancreatitis.
Dry skin and mucous membranes, increased intraocular pressure, headache, dizziness, central nervous system excitation, urinary retention.
Treatment: gastric lavage followed by the use of activated charcoal, symptomatic therapy, administration of methionine 8-9 hours after overdose and N-acetylcysteine 12 hours after (as antidotes to paracetamol), monitoring of the respiratory and circulatory systems (adrenaline should not be used). In case of convulsions, diazepam should be administered.
Adverse reactions
Caused by paracetamol.
From the digestive tract: rarely - nausea, vomiting, decreased appetite, constipation, diarrhea or flatulence, increased activity of liver enzymes, usually without the development of jaundice, hepatonecrosis (dose-dependent effect). With long-term use of significant doses of the drug - pain in the epigastric region, hepatotoxic effect.
From the blood system: very rarely - hemolytic anemia, sulfhemoglobinemia and methemoglobinemia (cyanosis, shortness of breath, heart pain), thrombocytopenia; in isolated cases - aplastic anemia, pancytopenia, neutropenia, agranulocytosis, leukopenia.
From the urinary system: renal colic, aseptic pyuria, interstitial glomerulonephritis, very rarely - nephrotoxic effect, papillary necrosis.
Allergic reactions: rarely - skin rashes, rashes on the mucous membranes (usually generalized rash, erythematous rash, urticaria), itching, hyperemia; very rarely - bronchial obstruction, exudative erythema multiforme (including Stevens-Johnson syndrome), toxic epidermal necrolysis (Lyell's syndrome); in isolated cases - anaphylactic shock, angioedema.
From the side of the central nervous system: (usually develops when taking high doses): dizziness, psychomotor agitation and disorientation.
On the part of the endocrine system: in rare cases - hypoglycemia, up to hypoglycemic coma.
Respiratory system: bronchospasm in patients sensitive to aspirin and other nonsteroidal anti-inflammatory drugs.
Others: in rare cases - general weakness, increased sweating.
Caused by dicyclomine hydrochloride.
Skin and subcutaneous tissue disorders: allergic reactions, skin redness.
On the part of the digestive tract: nausea, dry mouth, taste disturbance, thirst, dyspepsia, constipation, anorexia, increased activity of liver enzymes, usually without the development of jaundice, hepatonecrosis (dose-dependent effect), vomiting, abdominal pain, flatulence.
On the part of the organs of vision: pupil dilation with loss of accommodation and sensitivity to light, increased intraocular pressure, blurred vision, diplopia, mydriasis, cycloplegia of vision (paralysis of accommodation).
From the side of the central nervous system: dizziness, drowsiness, headache, paresthesia, sensory disturbances, nervousness, dyskinesia, lethargy, insomnia, general weakness, increased fatigue, syncope (loss of consciousness), numbness, gait disturbance.
Allergic reactions: skin itching, skin rashes, hives, urticaria, dry skin and other dermatological manifestations, severe allergic reactions or drug idiosyncrasy, including anaphylaxis.
Cardiovascular system: temporary bradycardia, tachycardia, arrhythmia, palpitations, hot flashes.
Urinary system disorders: urinary incontinence, urinary retention, impotence.
Mental disorders: speech disorders, confusion and/or emotional agitation, hallucinations, mood swings.
Musculoskeletal and connective tissue disorders: muscle weakness.
On the part of the respiratory system and chest organs: dyspnea, apnea, asphyxia, nasal congestion, sneezing, throat hyperemia.
Endocrine disorders: suppression of lactation.
Expiration date
3 years.
Storage conditions
Keep out of reach of children.
Store in the original packaging at a temperature not exceeding 30 °C.
Packaging
10 tablets in a blister, 10 blisters in a box.
Vacation category
According to the recipe.
Producer
Flamingo Pharmaceuticals Ltd.
Location of the manufacturer and its business address
E-28, Opp. Fire Brigade, M.I.D.S., Taloja, Raigad District, Maharashtra, IN– 410208, India.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.