Strepsils for children 6+ sugar-free lollipops blister No. 24
Instructions for Strepsils for children 6+ sugar-free lozenges blister No. 24
Composition
active ingredients: 2,4-dichlorobenzyl alcohol, amylmetacresol;
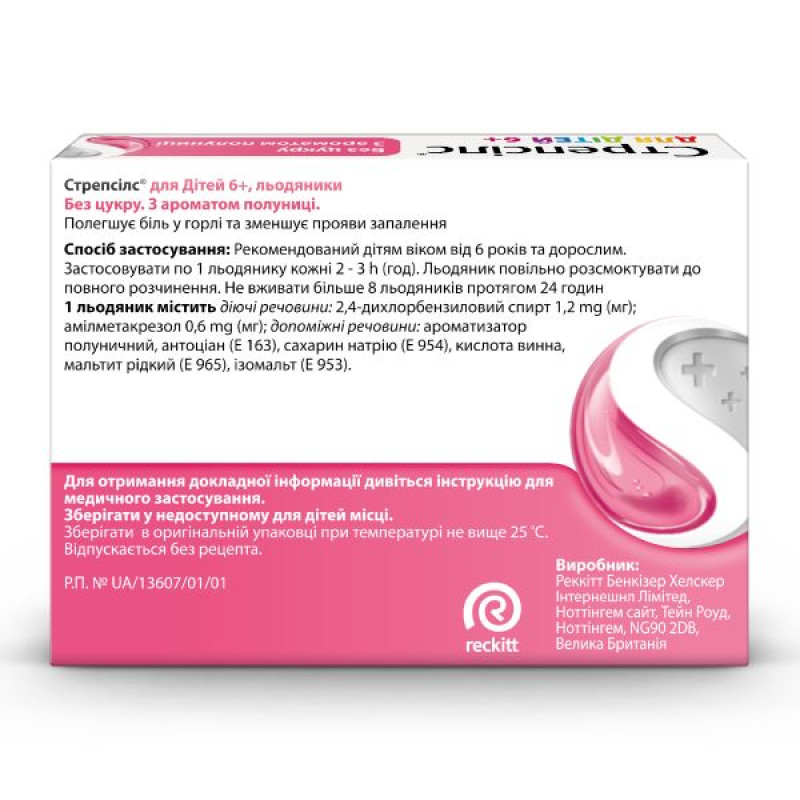
1 lollipop contains: 2,4-dichlorobenzyl alcohol 1.2 mg; amylmetacresol 0.6 mg;
excipients: strawberry flavoring, anthocyanin (E 163), sodium saccharin
(E 954), tartaric acid, liquid maltitol (E 965), isomalt (E 953).
Dosage form
Lollipops.
Main physicochemical properties: pink round lollipop with a characteristic strawberry aroma with an S sign on both sides.
Pharmacotherapeutic group
Medicines used for throat diseases. Antiseptics. Dichlorobenzyl alcohol. ATX code R02A A03.
Pharmacological properties
Pharmacodynamics.
The drug has antiseptic properties (active against a wide range of gram-positive and gram-negative microorganisms), has antifungal effects, has antiviral properties. The effectiveness of the drug is due to the presence of two broad-spectrum antibacterial components that relieve sore throat and reduce inflammation. Amylmetacresol destroys the structure of bacterial proteins, which provides a bactericidal effect. 2,4-dichlorobenzyl alcohol has a bacteriostatic effect due to dehydration of the bacterial cell. Both amylmetacresol and 2,4-dichlorobenzyl alcohol reversibly block ion channels similar to local anesthetics, and exhibit analgesic properties.
Pharmacokinetics.
Data missing
Indication
As an antiseptic to relieve acute sore throat.
Contraindication
Hypersensitivity to any component of the drug.
Interaction with other medicinal products and other types of interactions
Data is missing.
Application features
If symptoms persist for 3 days or worsen, you should consult a doctor for further adjustment of the treatment regimen.
Patients with rare hereditary diseases accompanied by fructose intolerance, glucose-galactose malabsorption or sucrose-isomaltase insufficiency should not use this drug.
The drug contains isomalt and liquid maltitol, which may have a mild laxative effect if multiple doses of the drug are taken throughout the day.
Use during pregnancy or breastfeeding
Pregnancy: There are no or limited amount of data from the use of amylmetacresol and 2,4-dichlorobenzyl alcohol. As with all medicines, caution should be exercised when using this medicine during pregnancy and if necessary, seek medical advice.
Breast-feeding: It is not known whether 2,4-dichlorobenzyl alcohol, amylmetacresol or metabolites are excreted in human milk. A risk to the newborn/infants cannot be excluded.
Fertility: There are no data on the effect on fertility.
Ability to influence reaction speed when driving vehicles or other mechanisms
The effect on the ability to drive or operate other mechanisms is absent or insignificant.
Method of administration and doses
For oral use. Recommended for adults and children over 6 years of age. Use 1 lollipop every 2 - 3 hours. Slowly dissolve the lollipop until completely dissolved. Recommended for use for 3 days. Do not use more than 8 lollipops in 24 hours. If symptoms persist or worsen, consult a doctor for additional adjustment of the treatment regimen.
No dose adjustment is necessary for elderly patients.
Children: Not recommended for use in children under 6 years of age.
Overdose
In the unlikely event of overdose, serious side effects are not expected. Some discomfort from the gastrointestinal tract may occur. Treatment is symptomatic.
Side effects
The following adverse reactions have been observed with short-term use:
2,4-dichlorobenzyl alcohol and amylmetacresol in non-prescription doses. Other adverse reactions may occur in the treatment of chronic diseases and with prolonged use.
Adverse reactions are classified by system organ class and frequency. The frequency is defined as follows: very common: ≥1/10; common: ≥1/100 and <1/10; uncommon: ≥1/1,000 and <1/100; rare: ≥1/10,000 and <1/1,000; very rare: <1/10,000; not known (cannot be estimated from the available data). Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness.
From the immune system.
Uncommon: hypersensitivity reactions, including rash, urticaria and angioedema, which may include swelling of the face, neck, throat or tongue, which may affect breathing.
From the respiratory tract and mediastinal organs.
Uncommon: pharyngeal oedema (swelling of the throat).
From the digestive tract.
Uncommon: oral discomfort (burning sensation in the mouth), throat irritation (burning sensation in the throat), oral paraesthesia (tingling sensation in the mouth), oral oedema. Rare: glossodynia (tongue pain). Very rare: dyspepsia, nausea, stomatitis. Not known: abdominal pain.
Not known: skin rash, itching, erythema.
The drug contains anthocyanin dye (E 163), which may cause allergic reactions.
Reporting of suspected adverse reactions.
It is important to report suspected adverse reactions during the post-marketing surveillance period. This allows for further monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals should be informed of any suspected adverse reactions.
Expiration date
2 years.
Storage conditions
Store in the original packaging at a temperature not exceeding 25 °C.
Keep out of reach of children.
Packaging
12 lozenges in a blister; 1 or 2 blisters in a cardboard box.
Vacation category
Without a prescription.
Producer
Reckitt Benckiser Healthcare International Limited.
Address
Nottingham site, Thane Road, Nottingham, NG90 2DB, United Kingdom.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.