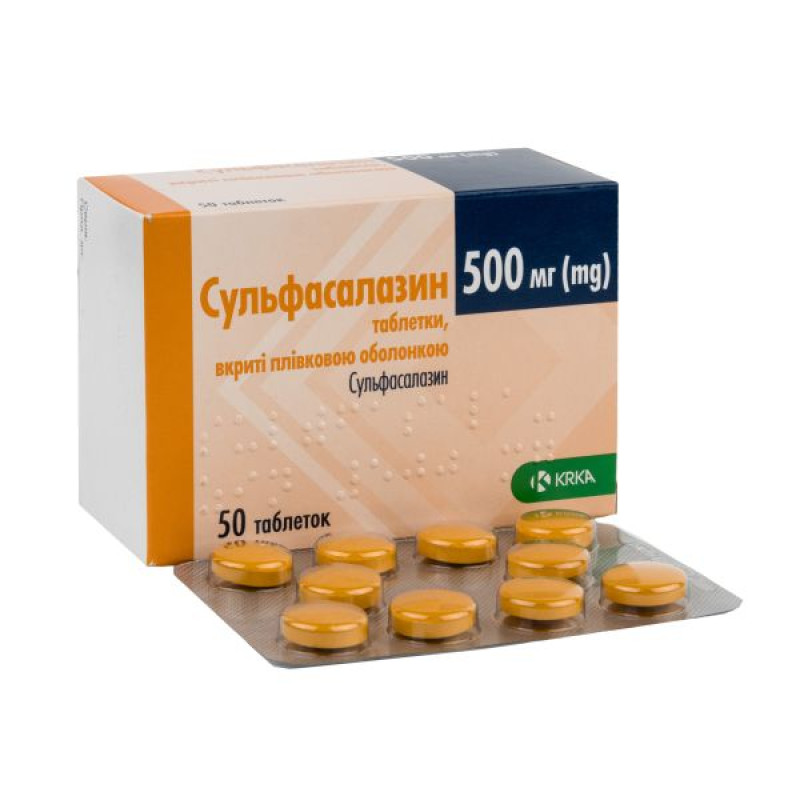
Sulfasalazine film-coated tablets 500 mg No. 50

Instructions Sulfasalazine film-coated tablets 500 mg No. 50
Composition
active ingredient: sulfasalazine;
1 tablet contains sulfasalazine 500 mg;
Excipients: povidone, pregelatinized starch, magnesium stearate, colloidal anhydrous silicon dioxide, hypromellose, propylene glycol.
Dosage form
Film-coated tablets.
Main physicochemical properties: round, brownish-yellow, slightly biconvex tablets with beveled edges, covered with a transparent colorless film coating.
Pharmacotherapeutic group
Anti-inflammatory drugs used in intestinal diseases. Aminosalicylic acid and similar drugs.
ATX code A07E C01.
Pharmacological properties
Pharmacodynamics
Sulfasalazine is an anti-inflammatory agent. It has an immunosuppressive effect, especially in the connective tissue, intestinal wall and serous fluid, where its concentration is highest. Thanks to the intestinal flora, sulfasalazine breaks down to sulfapyridine and 5-aminosalicylic acid. Sulfapyridine inhibits the proliferation of killer cells and the transformation of lymphocytes. The anti-inflammatory effect of 5-aminosalicylic acid (mesalazine) is most significant for the treatment of inflammatory diseases of the large intestine. It mainly inhibits cyclooxygenase and lipoxygenase locally in the intestinal wall, thereby preventing the formation of prostaglandins, leukotrienes and other inflammatory mediators. It is also likely to bind free oxygen radicals.
Pharmacokinetics
Approximately 30% of an administered dose of sulfasalazine is absorbed in the small intestine, the remaining 70% is metabolized by the intestinal flora in the large intestine to sulfapyridine and 5-aminosalicylic acid. The maximum plasma concentrations of sulfasalazine and its metabolites vary considerably between patients - with low levels of acetylation they are much higher and are associated with a higher incidence of adverse events. It is highly bound to plasma proteins and connective tissue. Most of the absorbed sulfasalazine is excreted in the bile into the intestine; a small amount is excreted unchanged in the urine. The half-life of sulfasalazine is 5 to 10 hours.
The majority of the released sulfapyridine is absorbed and reaches peak serum concentrations 12-24 hours after administration. It is metabolized in the liver (by acetylation, hydroxylation, and conjugation with glucuronic acid) and excreted by the kidneys. The half-life is 6 to 14 hours, depending on the rate of acetylation. Only about 30% of 5-aminosalicylic acid is absorbed and acetylated in the liver and excreted by the kidneys in the urine. The remainder is excreted unchanged in the feces.
Indication
– Induction and maintenance of remission in ulcerative colitis; treatment of Crohn's disease in the active stage.
– Treatment of rheumatoid arthritis in adults when nonsteroidal anti-inflammatory drugs (NSAIDs) are ineffective.
– Treatment of juvenile polyarticular or oligoarticular rheumatoid arthritis.
Contraindication
– Hypersensitivity to sulfasalazine, its metabolites, sulfonamides or salicylates.
– Intestinal obstruction or urinary tract obstruction.
– Porphyria, as sulfonamides have been reported to precipitate during an acute attack.
– Severe renal impairment (glomerular filtration rate < 30 ml/min/1.73m2) and/or severe hepatic impairment.
– Patients with a history of severe asthma attacks, urticaria, rhinitis or other allergic reactions caused by acetylsalicylic acid or other NSAIDs. Fatal anaphylactic reactions have been reported in such patients.
– Children under 6 years of age.
Interaction with other medicinal products and other types of interactions
There has been a decrease in the absorption of folic acid and digoxin when they are used concomitantly with sulfasalazine.
Bone marrow suppression and leukemia have been reported with concomitant use of the thiopurine 6-mercaptopurine or its prodrug, azathiopurine, with sulfasalazine (oral administration).
Coadministration of daily doses of sulfasalazine 2 g and weekly doses of methotrexate 7.5 mg in 15 patients with rheumatoid arthritis (in a drug interaction study) did not alter the pharmacokinetics of these drugs.
Daily doses of sulfasalazine 2 g (maximum 3 g) and weekly doses of methotrexate 7.5 mg (maximum 15 mg) were administered as monotherapy or in combination in 310 patients with rheumatoid arthritis in two controlled 52-week clinical trials. The overall toxicity profile of this combination showed an increased incidence of gastrointestinal adverse events, particularly nausea, compared with the incidence observed with the use of these drugs alone.
Laboratory Tests: There have been several reports of possible interference with laboratory test results (liquid chromatography) by urinary normetanephrine, resulting in false-positive results in patients taking sulfasalazine or its metabolite, mesalamine/mesalazine.
Application features
Sulfasalazine is particularly indicated in patients with ulcerative colitis who cannot take uncoated sulfasalazine tablets due to gastrointestinal intolerance and who have evidence that this intolerance is not primarily due to high blood levels of sulfapyridine and its metabolites, such as in patients who experience nausea and vomiting with the first few doses of the drug or in patients in whom dose reduction has not reduced gastrointestinal side effects. Patients with rheumatoid arthritis or juvenile rheumatoid arthritis should continue to maintain a regimen of rest and physical therapy as indicated. Unlike anti-inflammatory drugs, the effect of Sulfasalazine is not immediate. Concomitant treatment with analgesics and/or NSAIDs is recommended at least until the drug has taken effect.
Liver failure and elevated serum enzymes have been reported during treatment with 5-aminosalicylic acid/mesalazine derivatives in patients with a history of liver disease. Therefore, sulfasalazine is contraindicated in patients with severe liver disease (see section 4.3). Caution should be exercised when using the drug in patients with mild to moderate liver disease and the drug should be used only if the benefit clearly outweighs the risk to the patient. Liver function should be monitored before initiating therapy and periodically during treatment. Reports of renal injury, including minimal change nephropathy and chronic interstitial nephritis, have been associated with the use of mesalamine and its prodrugs. Sulfasalazine is contraindicated in patients with severe renal disease (see section 4.3). Caution should be exercised when using the drug in patients with mild to moderate renal impairment and the drug should be used only if the benefit of use significantly outweighs the risk to the patient. Renal function should be monitored before starting therapy and periodically during treatment. Fatal cases due to hypersensitivity reactions, agranulocytosis, aplastic anemia, other blood dyscrasias, liver and kidney damage, irreversible changes in the neuromuscular and central nervous systems, and fibrosing alveolitis have been reported in association with the use of sulfasalazine. The presence of clinical symptoms such as sore throat, fever, pallor, purpura, or jaundice may be signs of serious blood disorders or hepatotoxicity. Patients receiving Sulfasalazine should have a complete blood count and urinalysis with careful microscopic examination. Sulfasalazine treatment should be discontinued while awaiting the results of blood tests.
Oligospermia and infertility may occur in men treated with sulfasalazine. These effects are reversible upon discontinuation of the drug.
within 2-3 months.
Serious infections, including fatal sepsis and pneumonia, have been reported. Some infections have been associated with agranulocytosis, neutropenia or myelosuppression. If a patient develops a serious infection, the drug should be discontinued. Patients should be closely monitored for signs and symptoms of infection during and after treatment with the drug. A patient who develops a new infection while on treatment with the drug should undergo prompt and complete diagnostic workup to detect infection and myelosuppression. Caution should be exercised when considering the use of sulfasalazine in patients with a history of recurrent or chronic infections or with concomitant diseases or concomitant medications that may predispose patients to infections.
Severe hypersensitivity reactions may affect internal organs, causing hepatitis, nephritis, myocarditis, mononucleosis-like syndrome (pseudomononucleosis), blood abnormalities (including hematophagic histiocytosis), and/or pneumonitis, including eosinophilic infiltration.
Serious skin reactions, some fatal, including exfoliative dermatitis, Stevens-Johnson syndrome, and toxic epidermal necrolysis, have been reported with sulfasalazine. Patients are at highest risk of developing these events early in therapy, with the majority occurring within the first month of treatment.
Sulfasalazine should be discontinued at the first appearance of skin rash, mucosal lesions, or other signs of hypersensitivity.
Patients with hypersensitivity to furosemide, thiazide diuretics, carbonic anhydrase inhibitors should be monitored for signs of skin rash, mucosal lesions, or other manifestations of allergic reactions due to possible hypersensitivity to sulfasalazine in such patients.
Precautions.
General. The drug should be prescribed with caution to patients with severe allergies or bronchial asthma. To prevent crystalluria and stone formation, it is necessary to ensure that a sufficient amount of fluid enters the body. Patients with glucose-6-phosphate dehydrogenase deficiency should be carefully observed for signs of hemolytic anemia. This reaction is usually dose-dependent. If toxic reactions or hypersensitivity reactions occur, treatment with the drug should be discontinued immediately.
Information for patients.
Patients should be informed of the possibility of side effects and the need for close medical observation. The appearance of sore throat, fever, pallor, purpura, or jaundice may indicate a serious blood disorder. If any of these reactions occur, the patient should seek medical attention.
Patients should be instructed to take the drug in two equal doses. The tablets should be swallowed whole, preferably with food. Sulfasalazine may cause urine or skin to turn orange-yellow.
Ulcerative colitis: Patients with ulcerative colitis should be aware that ulcerative colitis rarely resolves completely, and the risk of exacerbations may be significantly reduced after long-term use of Sulfasalazine at a maintenance dose.
Rheumatoid arthritis. Rheumatoid arthritis rarely resolves completely. Therefore, long-term use of the drug is indicated. Physicians should monitor patients who require sulfasalazine to determine the need for long-term use of the drug.
Laboratory tests. Before starting Sulfasalazine, complete blood count with differential and liver function tests should be performed every 2 weeks for the first 3 months of therapy. During the next 3 months, the same tests should be performed once a month, and then every 3 months and as clinically indicated. Also, periodically during treatment with Sulfasalazine, complete urine analysis and assessment of renal function should be performed (see the section “Interference with laboratory test results” below).
It may be advisable to measure serum sulfapyridine levels, as concentrations above 50 μg/mL are likely to be associated with an increased incidence of adverse reactions.
Sulfasalazine, when administered orally, inhibits the absorption and metabolism of folic acid, which can cause its deficiency in the body (see section "Use during pregnancy or breastfeeding") and, in turn, potentially affect the development of serious blood disorders such as macrocytosis and pancytopenia.
Impact on laboratory test results
There have been several reports of possible interference of sulfasalazine or its metabolite mesalamine/mesalazine with the liquid chromatography assay for normetanephrine in urine, resulting in a false-positive test result.
Sulfasalazine or its metabolites may interfere with the absorption of ultraviolet light, especially at 340 nm, and may interfere with some laboratory tests that use NAD(H) or NADP(H) to measure the absorption of ultraviolet light around this wavelength. Examples of such tests include urea, ammonia, LDH, α-HBDH, and glucose. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), muscle/brain creatine kinase (CK-MB), glutamate dehydrogenase (GLDH), or thyroxine may be affected during high-dose sulfasalazine therapy. The laboratory should be consulted regarding the methodology used. This should be taken into account when evaluating laboratory test results if the patient is receiving sulfasalazine. The results should be interpreted in conjunction with the clinical data.
Use during pregnancy or breastfeeding
Pregnancy
Published data on the use of sulfasalazine in pregnant women indicate no teratogenic risk. The likelihood of adverse effects on the fetus when sulfasalazine is used during pregnancy is low. When administered orally, sulfasalazine inhibits the absorption and metabolism of folic acid and may lead to folic acid deficiency. Since harmful effects cannot be completely excluded, sulfasalazine should be used during pregnancy only if clearly needed.
Breastfeeding period
Sulfasalazine and sulfapyridine are excreted in breast milk. Caution should be exercised when administering the drug to nursing mothers, especially to premature infants or those with glucose-6-phosphate dehydrogenase deficiency. The decision on breastfeeding should be made by the physician, taking into account the benefit/risk ratio for the mother and child.
The ability to influence the reaction speed when driving or working with other mechanisms
The effect of sulfasalazine on the reaction rate when driving or operating other mechanisms has not been systematically evaluated.
Method of administration and doses
The dose should be adjusted according to the severity of the disease and possible side effects. The tablets should be taken during meals with a glass of liquid. The missed dose should be taken as soon as possible until it is almost time for the next dose. In this case, the patient should take only the next scheduled dose.
The tablets should be swallowed whole, not broken or crushed.
Elderly patients: no special precautions.
Ulcerative colitis
Adults
Severe: 2-4 tablets of Sulfasalazine 4 times a day, possibly in combination with steroids as part of an intensive care regimen. The effectiveness of the drug may decrease if the tablets are taken too quickly.
The nighttime interval between doses should not exceed 8 hours.
Moderate severity: 2-4 tablets 4 times a day, possible use in combination with steroids.
Mild course: 2 tablets 4 times a day with or without steroids.
Maintenance therapy: after induction of remission, the dose of the drug should be gradually reduced to 4 tablets per day. At this dose, the drug must be taken continuously, since when treatment is discontinued, even several years after an acute attack, the risk of relapse increases 4 times.
Children
The dose should be reduced in proportion to body weight.
In case of acute attack or relapse: 40-60 mg/kg per day.
Maintenance treatment: 20-30 mg/kg per day.
Crohn's disease
In Crohn's disease, the drug should be taken according to the same regimen as in ulcerative colitis (see above).
Rheumatoid arthritis
Adults
Patients with rheumatoid arthritis and those who have used NSAIDs for a long time may have a sensitive stomach, so in this case, Sulfasalazine should be used according to the following recommendations. Treatment should be started with 1 tablet per day, gradually increasing the dose by 1 tablet per day every week until the dose is 1 tablet 4 times a day or 2 tablets 3 times a day, depending on the tolerability and effectiveness of the drug. The effect is slow, and a pronounced effect may not be observed for 6 weeks. Improvement in joint mobility should be accompanied by a decrease in the erythrocyte sedimentation rate and the level of C-reactive protein. Concomitant use of NSAIDs and Sulfasalazine is possible.
Juvenile polyarticular or oligoarticular rheumatoid arthritis.
Children aged 6 and over.
30-50 mg/kg/day, divided into 4 equal doses. Usually the maximum daily dose is 2000 mg/day. To reduce possible gastrointestinal intolerance, the drug should be started with ¼ of the planned maintenance dose, followed by an increase of ¼ each week until the maintenance dose is reached.
Children
Studies have been reported on the safety and efficacy of sulfasalazine in the treatment of signs and symptoms of juvenile rheumatoid arthritis with polyarthritic syndrome in patients with rheumatoid arthritis aged 6 to 16 years. Extrapolation of data from adult patients with rheumatoid arthritis to children with juvenile rheumatoid arthritis with polyarthritic syndrome is based on the similarity of the disease and the effectiveness of therapy in these two groups of patients. Published study results support the possibility of extrapolating the safety and efficacy of sulfasalazine in juvenile rheumatoid arthritis with polyarthritic syndrome (see section 4.8).
A high incidence of adverse events has been reported in patients with systemic juvenile rheumatoid arthritis. The use of the drug in children with systemic juvenile rheumatoid arthritis has frequently resulted in serum sickness-like reactions. These reactions have often been severe and have included fever, nausea, vomiting, headache, rash, and abnormal liver function tests. Sulfasalazine is not recommended for systemic juvenile rheumatoid arthritis.
Overdose
There is evidence that the frequency and severity of toxic reactions in overdose are directly related to the total serum concentration of sulfapyridine. Symptoms of overdose may include nausea, vomiting, stomach upset, and abdominal pain. In more severe cases, central nervous system symptoms may occur, including drowsiness, convulsions, etc. Serum sulfapyridine concentrations can be used to monitor recovery from overdose.
Patients with renal impairment are at increased risk of severe toxicity.
Instructions for overdose. According to indications - gastric lavage or induction of vomiting and use of laxatives. Alkalinization of urine. With normal kidney function, intensive saturation of the body with water is necessary. In the presence of oliguria, the amount of fluid and saline administered should be limited and appropriate treatment should be carried out. In case of complete blockage of the kidneys by crystals, catheterization of the ureters can be performed. The low molecular weight of sulfasalazine and its metabolites may contribute to their removal by dialysis.
Patients should be monitored for signs of methemoglobinemia or sulfhemoglobinemia and treated appropriately if these conditions are present.
Adverse reactions
The most common adverse reactions associated with the use of sulfasalazine in ulcerative colitis were anorexia, headache, nausea, vomiting, stomach upset, and reversible oligospermia. These reactions occurred in approximately one-third of patients. Adverse reactions such as pruritus, urticaria, rash, fever, Heinz body anemia, hemolytic anemia, and cyanosis occurred less frequently (1 in 30 patients or less). Experience suggests that the incidence of adverse reactions increases with daily doses of 4 g or more or with total serum sulfapyridine levels above 50 μg/mL.
The use of sulfasalazine in rheumatoid arthritis in adults was associated with similar adverse reactions, although the incidence of individual reactions was higher. In rheumatoid arthritis studies, the following adverse reactions were commonly reported: nausea (19%), dyspepsia (13%), rash (13%), headache (9%), abdominal pain (8%), vomiting (8%), fever (5%), dizziness (4%), stomatitis (4%), pruritus (4%), liver function test abnormal (4%), leukopenia (3%), and thrombocytopenia (1%). There was one report of a 10% level of immunoglobulin suppression. This reaction was slowly reversible and was rarely associated with clinical symptoms.
In general, adverse reactions in patients with juvenile rheumatoid arthritis are similar to those seen in adult patients with rheumatoid arthritis, with the exception of a high incidence of serum sickness-like syndrome in systemic juvenile rheumatoid arthritis. In one clinical trial, a 10% rate of immunoglobulin suppression was observed.
Although the list below only includes a small number of adverse reactions reported with this particular drug, the pharmacological similarity of sulfonamides suggests that each of these reactions should be considered when using Sulfasalazine.
Adverse reactions that occur infrequently or rarely
Infections and infestations: aseptic meningitis, pseudomembranous colitis.
From the blood and lymphatic system: pancytopenia, aplastic anemia, agranulocytosis, megaloblastic (macrocytic) anemia, purpura, hypoprothrombinemia, methemoglobinemia, macrocytosis, congenital neutropenia and myelodysplastic syndrome.
Immune system disorders: erythema multiforme (Stevens-Johnson syndrome), exfoliative dermatitis, epidermal necrolysis (Lyell's syndrome) with corneal damage, drug rash with eosinophilia and systemic symptoms (DRESS), anaphylaxis, serum sickness syndrome, interstitial lung disease, pneumonitis with or without eosinophilia, vasculitis, fibrosing alveolitis, pleurisy, pericarditis with or without tamponade, allergic myocarditis, polyarteritis nodosa, lupus-like syndrome, hepatitis and hepatic necrosis with or without immune complexes, fulminant hepatitis, sometimes leading to liver transplantation, acute pox-like parapsoriasis (Mucha-Habermann syndrome), rhabdomyolysis, photosensitization, arthralgia, periorbital edema, conjunctival and sclera injection and alopecia, reactions hypersensitivity.
Gastrointestinal: hepatitis, liver failure, pancreatitis, bloody diarrhea, impaired folic acid absorption, impaired digoxin absorption, stomatitis, diarrhea, abdominal pain and neutropenic enterocolitis, exacerbation of ulcerative colitis.
Mental disorders: depression.
Central nervous system: taste disorders, transverse myelitis, convulsions, meningitis, transverse lesion of the posterior spinal cord, cauda equina syndrome, Guillain-Barré syndrome, encephalopathy, peripheral neuropathy, depression of mental functions, dizziness, hearing loss, olfactory disorders, insomnia, ataxia, hallucinations, tinnitus and drowsiness.
Renal: toxic nephropathy with oliguria and anuria, nephritis, nephrotic syndrome, urinary tract infections, hematuria, crystalluria, proteinuria and hemolytic-uremic syndrome, interstitial nephritis.
Other reactions: change in urine color and change in skin color.
Sulfonamides have a certain chemical similarity to some vasopressors, diuretics (acetazolamide and thiazides), and oral hypoglycemic drugs. Patients receiving sulfonamides rarely experience goiter enlargement, hypoglycemia, and diuresis.
Cross-sensitivity may occur with these agents. Rats appear to be particularly sensitive to the stromogenic effects of sulfonamides, and long-term use of these agents in this species has resulted in malignant thyroid tumors.
The following are events that have been identified during post-marketing clinical use of products containing mesalamine (or metabolized to mesalamine). Since reports were submitted voluntarily from an unknown number of individuals, it is not possible to estimate their frequency. These events have been included due to a combination of factors such as seriousness, frequency of reporting, or potential causal relationship to mesalamine.
From the blood and lymphatic system: pseudomononucleosis.
Cardiac disorders: myocarditis.
Hepatobiliary: Hepatotoxicity, including elevations in liver function tests (AST/AST, ALT/AST, GGT, LDH, alkaline phosphatase, bilirubin), jaundice, cholestatic jaundice, cirrhosis, cholestatic hepatitis, cholestasis and possible hepatocellular injury including hepatic necrosis and hepatic failure, has been reported. Some of these cases have been fatal. One case of Kawasaki-like syndrome involving liver function abnormalities has been reported.
Immune system disorders: anaphylaxis.
Metabolism: loss of appetite, folate deficiency.
From the urinary system: nephrolithiasis.
Respiratory, thoracic and mediastinal disorders: cough, dyspnea, oropharyngeal pain.
Skin and subcutaneous tissue disorders: angioedema, purpura, exanthema, toxic pustuloderma, lichen planus, photosensitivity, Gougereau-Sjogren's syndrome.
Vascular disorders: pallor.
Drug abuse and dependence: Not reported.
Laboratory tests: increased liver enzymes, induction of autoantibodies.
Expiration date
5 years.
Storage conditions
Store at a temperature not exceeding 25 ° C. Keep out of the reach of children.
Packaging
10 tablets in a blister; 5 blisters in a cardboard box.
Vacation category
According to the recipe.
Producer
KRKA, dd, Novo mesto/KRKA, dd, Novo mesto.
Location of the manufacturer and address of its place of business
Smarjeska cesta 6, 8501 Novo mesto, Slovenia.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.