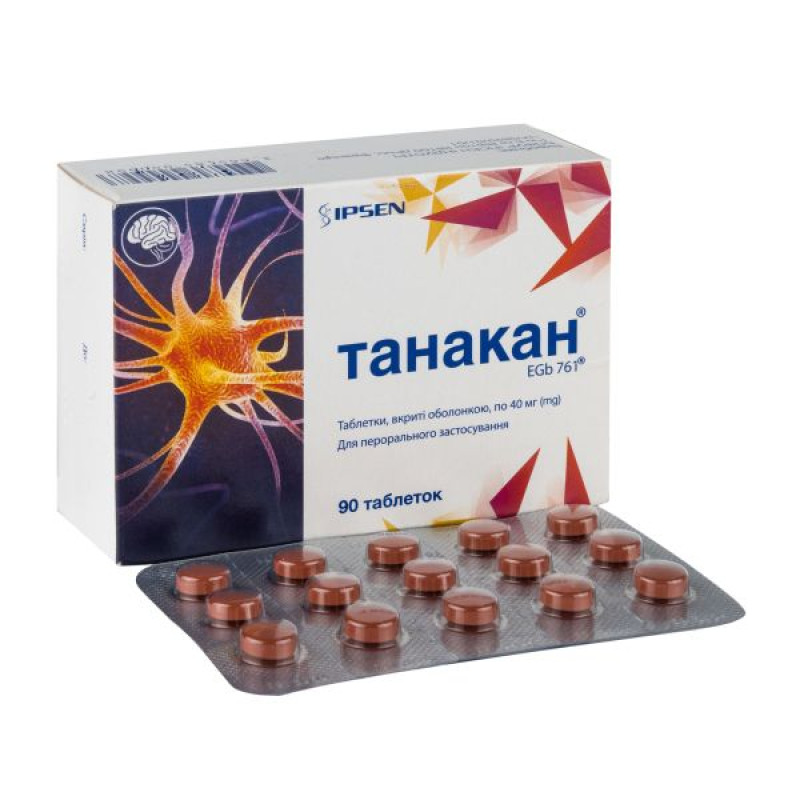
Tanakan film-coated tablets 40 mg No. 90
Instructions for Tanakan film-coated tablets 40 mg No. 90
Composition
active ingredient: 1 tablet contains Ginkgo biloba dry extract (EGb 761) 40 mg;
excipients: lactose monohydrate, microcrystalline cellulose, corn starch, colloidal anhydrous silicon dioxide, talc, magnesium stearate;
composition of the tablet shell: macrogol 400, macrogol 6000, hypromellose, titanium dioxide
(E 171), iron oxide red (E 172).
Dosage form
Film-coated tablets.
Main physicochemical properties: round biconvex tablets, coated with a dark red shell, the core has a light brown color and a specific odor when broken.
Pharmacotherapeutic group
Dementia treatment.
ATX code N06D X02.
Pharmacological properties
Pharmacodynamics
The numerical mechanisms underlying the therapeutic effect have not yet been studied in humans.
Pharmacokinetics
Active ingredient: standardized extract of Ginkgo biloba: 24% heterosides and 6% di- and sesquiterpenes (ginkgolides A, B and C and bilobalide).
In humans, only the pharmacokinetic parameters of the terpene fraction have been described.
The oral bioavailability of ginkgolides A and B and bilobalides is
80-90%. Peak concentrations are reached within 1-2 hours; half-lives range from approximately 4 hours (bilobalide, ginkgolide A) to 10 hours (ginkgolide B).
These substances do not break down in the body and are almost completely excreted in the urine, and a small amount is excreted in the feces.
Indication
- Symptomatic treatment of cognitive disorders in elderly patients, with the exception of patients with confirmed dementia, Parkinson's disease, cognitive disorders of iatrogenic origin or those arising as a result of complications of depression, vascular disorders, metabolic disorders.
- Concomitant treatment of vertigo of vestibular origin together with vestibular rehabilitation.
- Symptomatic treatment of ringing in the ears.
Contraindication
Hypersensitivity to any component of the drug.
Interaction with other medicinal products and other types of interactions
Results of clinical interaction studies with Ginkgo biloba (EGb 761) have shown potentiation or inhibition of cytochrome P450 isoenzymes. Midazolam concentrations were altered after concomitant administration of Ginkgo biloba (EGb 761), suggesting an interaction via CYP3A4. Therefore, medicinal products that are primarily metabolised by CYP3A4 and have a narrow therapeutic index should be used with caution.
Application features
It is recommended to closely monitor patients who are taking concomitant medications that are metabolized by cytochrome P450 3A4. There is no information on the abuse of Ginkgo biloba (EGb 761). Based on the pharmacological characteristics of the drug, Ginkgo biloba (EGb 761) has no potential for abuse.
The drug contains lactose, therefore patients with rare hereditary forms of galactose intolerance, lactase deficiency or glucose-galactose malabsorption syndrome should not use the drug.
Use during pregnancy or breastfeeding
This medicine should be used primarily in elderly patients who are not at risk of pregnancy.
Due to the lack of relevant clinical data, the use of this product is not recommended during pregnancy or breastfeeding.
Ability to influence reaction speed when driving vehicles or other mechanisms
No studies have been conducted to assess the effect on the reaction speed when driving or using other mechanisms. However, dizziness may impair the ability to drive or use other mechanisms.
Method of administration and doses
For oral use.
Take 1 tablet 3 times a day with meals. Wash down with half a glass of water.
The course of treatment is determined by the doctor individually.
Children
Do not use on children.
Overdose
There is no information on overdose of the drug.
Adverse reactions
The following reactions are occasionally observed:
-from the digestive system: digestive disorders; dyspepsia; diarrhea; abdominal pain; nausea, vomiting;
- from the immune system: hypersensitivity reactions, including angioedema, urticaria, shortness of breath;
-skin: skin inflammation, redness, swelling, rash, itching, eczema;
- from the nervous system: headache; dizziness; syncope (including vasovagal).
Expiration date
36 months.
Storage conditions
Store in original packaging at a temperature not exceeding 25 0C.
Keep out of reach of children.
Packaging
15 tablets in a blister, 2 or 6 blisters in a cardboard box.
Vacation category
Without a prescription.
Producer
BOFUR IPSAIN INDUSTRIES.
Location of the manufacturer and its business address
Rue Ete Virton 28100 Droit, France.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.