Tenzocard modified-release capsules 10 mg/1.5 mg No. 30
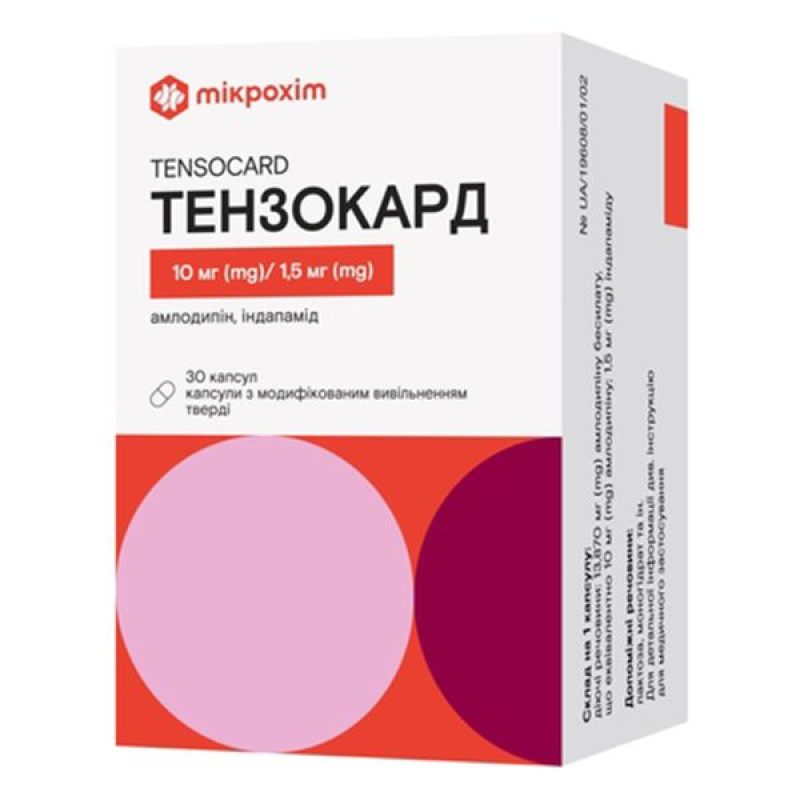
Instructions for Tenzocard modified-release capsules 10 mg/1.5 mg No. 30
Composition
active ingredients: amlodipine, indapamide;
1 capsule contains:
6.935 mg amlodipine besylate, equivalent to 5 mg amlodipine, and 1.5 mg indapamide,
or 13.870 mg amlodipine besylate, equivalent to 10 mg amlodipine, and 1.5 mg indapamide;
excipients:
for amlodipine tablets: lactose monohydrate; microcrystalline cellulose; crospovidone; magnesium stearate; colloidal anhydrous silica;
for indapamide tablets: lactose, monohydrate; hypromellose K4M; povidone; hypromellose E6; titanium dioxide (E 171); magnesium stearate; polyethylene glycol 6000 (macrogol 6000); iron oxide (E 172); colloidal anhydrous silicon dioxide;
capsule (body and cap): gelatin; water; titanium dioxide (E 171).
Dosage form
Modified-release capsules are hard.
Main physicochemical properties: opaque, hard gelatin capsules of white or almost white color, containing two white or almost white amlodipine tablets and one yellow-brown film-coated indapamide tablet.
Pharmacotherapeutic group
Calcium channel blockers and diuretics. Amlodipine and diuretics. ATC code C08G A02.
Pharmacological properties
Pharmacodynamics
Mechanism of action
Indapamide is a sulfonamide derivative with an indole ring, pharmacologically related to thiazide diuretics, which acts by inhibiting sodium reabsorption in the cortical segment of the kidney. This increases the urinary excretion of sodium and chloride and, to a lesser extent, potassium and magnesium, thereby increasing urine output and providing an antihypertensive effect.
Amlodipine is a calcium ion influx inhibitor of the dihydropyridine group (slow calcium channel blocker or calcium ion antagonist), which prevents the transmembrane influx of calcium ions into myocardial and vascular smooth muscle. The mechanism of antihypertensive action of amlodipine is its ability to relax vascular smooth muscle.
Pharmacodynamic effects
Clinical trials of phase II and III have shown that when indapamide is used as monotherapy, the antihypertensive effect lasts for 24 hours. This effect is manifested at doses in which diuretic properties are minimal. The antihypertensive effect of indapamide is associated with an improvement in arterial elasticity, a decrease in arteriolar resistance and total peripheral vascular resistance. Indapamide reduces left ventricular hypertrophy.
When the recommended dose is exceeded, the therapeutic effect of thiazide and thiazide-like diuretics does not increase, while the number of adverse reactions increases. In the absence of a treatment effect, the dose should not be increased.
Also, in the course of short-, medium- and long-term studies involving patients with arterial hypertension, it was demonstrated that indapamide:
- does not affect lipid metabolism (triglycerides, low- and high-density lipoproteins);
- does not affect carbohydrate metabolism, even in patients with diabetes mellitus and hypertension.
In patients with arterial hypertension, the use of amlodipine once a day provides a clinically significant reduction in blood pressure for 24 hours in both the supine and standing positions. Due to the slow onset of action of amlodipine, its use does not lead to acute hypotension. The use of amlodipine has not been associated with the occurrence of any metabolic adverse reactions or changes in plasma lipid levels, so it can be used in patients with asthma, gout and diabetes mellitus.
Pharmacokinetics
The simultaneous use of indapamide and amlodipine does not change their pharmacokinetic properties compared to their separate use.
Indapamide
Indapamide 1.5 mg is presented in a prolonged-release dosage form, which is provided by a matrix system in which the active substance is distributed and which enables uniform prolonged release of indapamide.
Absorption
Indapamide released from the tablet is rapidly and completely absorbed from the gastrointestinal tract. Food intake slightly increases the rate of absorption, but does not affect the amount of active substance absorbed. The maximum concentration in the blood plasma is reached approximately 12 hours after oral administration of a single dose, repeated administration reduces the fluctuations in the level of indapamide in the blood plasma between two doses of the drug. There is intra-individual variability.
Distribution
Indapamide is 79% bound to plasma proteins. The elimination half-life is 14 to 24 hours (average 18 hours). Steady-state concentrations are reached after 7 days. Repeated administration does not lead to accumulation.
Breeding
Excretion occurs mainly in urine (70% of the dose) and feces (22%) in the form of inactive metabolites.
High-risk patients
In patients with renal insufficiency, pharmacokinetic parameters do not change.
Amlodipine
Amlodipine is available in an immediate-release dosage form.
After oral administration in therapeutic doses, amlodipine is well absorbed, with peak blood concentrations occurring within 6–12 hours. Absolute bioavailability is 64–80%. The volume of distribution is approximately 21 l/kg. In vitro studies have shown that approximately 97.5% of circulating amlodipine is bound to plasma proteins. Food intake does not affect the bioavailability of amlodipine.
Biotransformation/excretion
The plasma half-life of amlodipine is approximately 35–50 hours, corresponding to a single daily dose. Amlodipine is extensively metabolized by the liver to inactive metabolites, excreted in the urine as unchanged drug (10%) and as metabolites (60%).
Use in patients with hepatic impairment
There is limited clinical data on the use of amlodipine in patients with hepatic impairment. In patients with hepatic insufficiency, the clearance of amlodipine is reduced, resulting in an increase in half-life and area under the pharmacokinetic concentration-time curve (AUC) by approximately 40-60%.
Use in elderly patients
The time to reach maximum plasma concentrations of amlodipine is similar in elderly and younger patients. There is a tendency for amlodipine clearance to decrease in elderly patients, resulting in an increase in AUC and half-life. In subjects with congestive heart failure, the increase in AUC and half-life was expected for the age group studied.
Indication
Treatment of essential hypertension in patients who require treatment with indapamide and amlodipine in doses available in fixed combinations.
Contraindication
- Hypersensitivity to the active substances, other sulfonamides, dihydropyridine derivatives or to any of the excipients of the medicinal product;
- severe renal failure (creatinine clearance
- hepatic encephalopathy or severe liver dysfunction;
- hypokalemia;
- breastfeeding period;
- severe hypotension;
- shock (including cardiogenic);
- obstruction of the outlet from the left ventricle (for example, severe aortic stenosis);
- heart failure with unstable hemodynamics after acute myocardial infarction.
Interaction with other medicinal products and other types of interactions
Interactions related to indapamide
Not recommended combinations
Lithium
It is possible to increase the concentration of lithium in the blood plasma with the appearance of symptoms of overdose, similar to those of a salt-free diet (reduced urinary lithium excretion). However, if the use of diuretics is really necessary, the level of lithium in the blood plasma should be carefully monitored and the dose of the diuretic should be adjusted.
Combinations requiring precautions for use
Drugs that can cause the development of paroxysmal ventricular tachycardia of the "pirouette" type:
· class IA antiarrhythmic drugs (quinidine, hydroquinidine, disopyramide);
· class III antiarrhythmic drugs (amiodarone, sotalol, dofetilide, ibutilide, bretylium);
· some antipsychotics:
- phenothiazines (chlorpromazine, cyamemazine, levomepromazine, thioridazine, trifluoperazine);
- benzamides (amisulpride, sulpiride, sultopride, tiapride);
- butyrophenones (droperidol, haloperidol);
- other antipsychotics (e.g. pimozide);
· other medicines: bepridil, cisapride, diphemanil, intravenous erythromycin, halofantrine, mizolastine, pentamidine, sparfloxacin, moxifloxacin, intravenous vincamine, methadone, astemizole, terfenadine.
The risk of ventricular arrhythmias, in particular torsades de pointes (hypokalemia is a risk factor), increases. Before prescribing this combination, check for hypokalemia and correct potassium levels if necessary. The patient's clinical condition, plasma electrolyte levels and ECG monitoring should be monitored. In the presence of hypokalemia, drugs that do not cause torsades de pointes should be used.
Nonsteroidal anti-inflammatory drugs (NSAIDs) (systemic use), including selective cyclooxygenase-2 inhibitors and acetylsalicylic acid in high doses (≥ 3 g per day)
With simultaneous use, the antihypertensive effect of indapamide may be weakened. In dehydrated patients, the risk of acute renal failure (decreased glomerular filtration) increases. Before starting treatment, water balance should be restored and renal function checked.
Angiotensin-converting enzyme (ACE) inhibitors
In the presence of sodium deficiency, treatment with ACE inhibitors may cause sudden arterial hypotension and/or acute renal failure (especially in patients with renal artery stenosis).
For patients with arterial hypertension in whom previous diuretic therapy has led to sodium deficiency, it is necessary to:
- 3 days before starting treatment with ACE inhibitors, stop taking the diuretic and then, if necessary, resume taking the diuretic;
For patients with congestive heart failure, the ACE inhibitor should be initiated at the lowest dose, possibly after reducing the dose of the concomitant potassium-sparing diuretic.
In all cases, renal function (plasma creatinine level) should be monitored during the first weeks of treatment with ACE inhibitors.
Other compounds that may cause hypokalemia: intravenous amphotericin B, gluco- and mineralocorticoids (systemic), tetracosactide, laxatives that stimulate peristalsis
The risk of hypokalemia increases (additive effect). The level of potassium in the blood plasma should be monitored and corrected if necessary. This should be especially remembered during concomitant treatment with cardiac glycosides. It is recommended to use laxatives that do not stimulate peristalsis.
Digitalis preparations
Decreased blood potassium levels increase the toxic effects of digitalis drugs. Blood potassium levels and ECG should be monitored and therapy reviewed if necessary.
Baclofen
The antihypertensive effect is increased. At the beginning of treatment, the patient's water balance should be restored and kidney function should be monitored.
Allopurinol
Concomitant use with indapamide may lead to an increased incidence of hypersensitivity reactions to allopurinol.
Combinations that require attention
Potassium-sparing diuretics (amiloride, spironolactone, triamterene)
Despite the rationality of prescribing this combination in some patients, hypokalemia or hyperkalemia may occur (especially in patients with renal insufficiency or diabetes mellitus). Plasma potassium levels should be monitored, ECG monitoring should be performed and, if necessary, therapy should be reviewed.
Metformin
The risk of metformin-induced lactic acidosis is increased due to the possible development of functional renal failure associated with the use of diuretics, especially loop diuretics. Metformin should not be prescribed if the level of creatinine in the blood plasma exceeds 15 mg / l (135 μmol / l) in men and 12 mg / l (110 μmol / l) in women.
Iodine contrast agents
In the presence of dehydration caused by the use of diuretics, the risk of developing acute renal failure increases, especially when using large doses of iodocontrast agents. Water balance should be restored before the use of iodocontrast agents.
Imipramine-like antidepressants, neuroleptics
The antihypertensive effect and the risk of developing orthostatic hypotension increase (additive effect).
Calcium (salts)
Hypercalcemia may occur due to decreased urinary calcium elimination.
Cyclosporine, tacrolimus
Plasma creatinine levels may increase without changing circulating cyclosporine levels, even in the absence of water and sodium depletion.
Corticosteroids, tetracosactide (systemic)
Possible reduction in antihypertensive effect (water and sodium retention under the influence of corticosteroids).
Interactions related to amlodipine
Dantrolene (infusion)
In animal studies, fatal ventricular fibrillation and cardiovascular collapse have been observed in association with hyperkalemia following intravenous administration of verapamil and dantrolene. In patients predisposed to malignant hyperthermia and in the treatment of malignant hyperthermia, it is recommended that concomitant use of calcium channel blockers such as amlodipine be avoided due to the risk of hyperkalemia.
Grapefruit or grapefruit juice
It is not recommended to use amlodipine with grapefruit or grapefruit juice, as bioavailability may increase in some patients, leading to increased hypotensive effect.
CYP3A4 inhibitors
Concomitant use of amlodipine with strong or moderate CYP3A4 inhibitors (protease inhibitors, azole antifungals, macrolides such as erythromycin or clarithromycin, verapamil or diltiazem) may result in significant increases in amlodipine concentrations. These pharmacokinetic changes are clinically more pronounced in elderly patients. Therefore, clinical monitoring and dose adjustment may be necessary. There is an increased risk of hypotension in patients receiving clarithromycin in combination with amlodipine. Close observation is recommended in such patients.
CYP3A4 inducers
When used concomitantly with known CYP3A4 inducers, the plasma concentration of amlodipine may change. Therefore, blood pressure should be monitored and dose adjustments should be made during and after concomitant use with CYP3A4 inducers, particularly strong CYP3A4 inducers (e.g. rifampicin, St. John's wort (hypericum perforatum)).
Effect of amlodipine on other medicinal products
The hypotensive effects of amlodipine potentiate the hypotensive effects of other medicinal products with antihypertensive properties (additive effect). Clinical interaction studies have shown that amlodipine does not affect the pharmacokinetics of atorvastatin, digoxin or warfarin.
Tacrolimus
Mechanistic target of rapamycin (mTOR) inhibitors
mTOR inhibitors such as sirolimus, temsirolimus and everolimus are substrates of CYP3A. Amlodipine is a weak CYP3A inhibitor. When used concomitantly with mTOR inhibitors, amlodipine may potentiate the effects of the latter.
Cyclosporine
No interaction studies of cyclosporine and amlodipine have been conducted in healthy volunteers or other patient groups, except in renal transplant patients, in whom an increase in the fluctuation of the trough concentration of cyclosporine (on average from 0 to 40%) was observed. In renal transplant patients receiving amlodipine, the level of cyclosporine in the blood should be monitored and, if necessary, its dose should be reduced.
Simvastatin
The use of amlodipine in a dose of 10 mg in combination with 80 mg of simvastatin resulted in 77% higher concentrations of simvastatin compared to its use as monotherapy. Patients using amlodipine should limit the dose of simvastatin to 20 mg per day.
Application features
Hepatic encephalopathy
In patients with impaired liver function, the use of thiazide-like diuretics may cause hepatic encephalopathy, which may progress to hepatic coma, especially in the case of electrolyte imbalance. In such cases, the use of TENZOKARD should be discontinued immediately due to the presence of indapamide in its composition.
Photosensitivity
Photosensitivity reactions have been reported with thiazide and thiazide-like diuretics (see section 4.8). If a photosensitivity reaction occurs during treatment, the drug should be discontinued. If re-administration is necessary, it is recommended to protect the affected areas from the sun or artificial ultraviolet light sources.
Precautions for use
Hypertensive crisis
Targeted studies on the safety and efficacy of amlodipine in hypertensive crisis have not been conducted.
Water and electrolyte balance:
Plasma sodium level
Before starting treatment and at regular intervals thereafter, serum sodium levels should be determined. A decrease in serum sodium levels may initially be asymptomatic, therefore monitoring is necessary. In elderly patients and those suffering from cirrhosis of the liver, monitoring should be carried out more frequently (see sections 4.8 and 4.8). Any diuretic treatment can cause hyponatremia, sometimes with very serious consequences. Hyponatremia in combination with hypovolemia can lead to dehydration and orthostatic hypotension. The concomitant loss of chloride ions can lead to secondary compensatory metabolic alkalosis; the frequency and severity of this effect are minor.
Plasma potassium level
A decrease in plasma potassium with hypokalaemia is the main risk with thiazide and thiazide-like diuretics. Hypokalaemia may cause muscle disorders. Rhabdomyolysis has been reported, mainly with severe hypokalaemia. Hypokalaemia (pirouette), which can be fatal, should be avoided. In all of the above cases, more frequent monitoring of plasma potassium is necessary. The first determination of plasma potassium should be performed within the first week of treatment. If hypokalaemia is detected, the plasma potassium level should be corrected. Hypokalaemia detected in association with low serum magnesium may be refractory to treatment unless serum magnesium is corrected.
Magnesium in blood plasma
Thiazides and related diuretics, including indapamide, have been shown to increase urinary magnesium excretion, which may lead to hypomagnesemia (see sections 4.5 and 4.8).
Plasma calcium level
Thiazide and thiazide-like diuretics may reduce urinary calcium excretion and lead to a slight and transient increase in plasma calcium levels. The occurrence of hypercalcemia may be associated with undiagnosed hyperparathyroidism. In such cases, treatment should be discontinued and parathyroid function should be monitored.
Plasma glucose level
Due to the presence of indapamide in the composition of the drug, monitoring of blood glucose levels is important for patients with diabetes, especially in the presence of hypokalemia.
Heart failure
Patients with heart failure should be prescribed TENZOKARD with caution. In a long-term placebo-controlled study in patients with severe heart failure (New York Heart Association functional class III and IV), the incidence of pulmonary edema was higher with amlodipine than with placebo. Calcium antagonists, including amlodipine, should be prescribed with caution in patients with congestive heart failure, as they increase the risk of cardiovascular events and death.
Kidney function
Patients with renal insufficiency can use amlodipine in usual doses. Fluctuations in plasma concentrations of amlodipine do not depend on the severity of renal insufficiency. Amlodipine is not dialyzable. Studies on the effectiveness of the combination of amlodipine/indapamide in patients with renal dysfunction have not been conducted. For patients with impaired renal function, the dose of the drug should be selected in accordance with the dosage of each individual component when used as monotherapy.
Uric acid level in blood plasma
In patients with a history of gout, there is a possibility of an increase in the number of gout attacks due to an increase in the level of uric acid in the blood plasma due to the presence of indapamide in the composition of the drug.
Patients with liver dysfunction
In patients with impaired liver function, amlodipine half-life is prolonged and AUC is increased, and no dosage recommendations are available. Therefore, amlodipine treatment should be initiated at the lowest dose. The drug should be used with caution at the beginning of treatment and when increasing the dose. Targeted studies of the efficacy of the amlodipine/indapamide combination in patients with impaired liver function have not been conducted. Due to the properties of indapamide and amlodipine, TENZOKARD is contraindicated in patients with severe liver function impairment. TENZOKARD should be used with caution in patients with mild to moderate liver function impairment.
Elderly patients
In elderly patients, the drug TENZOKARD should be used taking into account renal function (see sections “Pharmacokinetics” and “Method of administration and dosage”).
Choroidal effusion, acute myopia (nearsightedness), and secondary angle-closure glaucoma
Drugs containing sulfonamide or sulfonamide derivatives may cause an idiosyncratic reaction resulting in choroidal effusion with visual field defect, transient myopia, and acute angle-closure glaucoma. Symptoms include acute onset of decreased visual acuity or eye pain and usually occur within hours to weeks of starting the drug. Untreated acute angle-closure glaucoma may lead to permanent vision loss. The primary treatment is immediate discontinuation of the drug. If intraocular pressure remains uncontrolled, prompt medical or surgical treatment may be necessary. Risk factors for acute angle-closure glaucoma may include a history of sulfonamide or penicillin allergy.
Athletes
Athletes should take into account that the drug contains an active substance that may cause a positive reaction in doping control.
Excipients
Since the drug TENZOKARD contains lactose, it should not be administered to patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption.
Use during pregnancy or breastfeeding
Considering the effect of the components of the combined medicinal product TENZOKARD on the course of pregnancy and breastfeeding:
- the use of the drug during pregnancy is not recommended;
- the drug is contraindicated during breastfeeding.
Pregnancy
Precautions related to indapamide
There are no or limited (less than 300) data on the use of indapamide in pregnant women. Prolonged use of a thiazide diuretic during the third trimester of pregnancy may result in a decrease in circulating blood volume (CVD) of the pregnant woman and uteroplacental blood flow, which may cause fetoplacental ischemia and fetal growth retardation. In addition, in rare cases, hypoglycemia and thrombocytopenia have been observed in the newborn. Animal studies do not indicate direct or indirect toxic effects with respect to reproductive function.
Precautions related to amlodipine
Studies on the safety of amlodipine in pregnant women have not been conducted. In animal studies, reproductive toxicity was observed at high doses.
Breast-feeding
Precautions related to indapamide
There is insufficient data on the excretion of indapamide/metabolites in breast milk. Hypersensitivity to sulfonamide derivatives and hypokalemia may develop. A risk to the newborn/infants cannot be excluded. Indapamide belongs to the thiazide-like diuretics, the use of which during breastfeeding has been associated with a decrease or even suppression of lactation.
Precautions related to amlodipine
Amlodipine is excreted in human milk. The proportion of the initial maternal dose received by the infant was estimated to be in the interquartile range of 3–7% with a maximum of 15%. The effects of amlodipine on infants are unknown.
Fertility
Precautions related to indapamide
Reproductive toxicity studies showed no effect on fertility in male and female rats. No effect on human fertility is expected.
Reversible biochemical changes in the head of sperm have been observed in some patients receiving calcium channel blockers. Clinical data on the potential effect of amlodipine on fertility are insufficient. In one animal study, adverse reactions affecting male fertility were observed.
Ability to influence reaction speed when driving vehicles or other mechanisms
The drug TENZOKARD has minor or moderate influence on the ability to drive and use machines.
Indapamide
Indapamide does not affect alertness, but in individual cases various reactions associated with a decrease in blood pressure may occur, especially at the beginning of treatment or when used simultaneously with other antihypertensive drugs. As a result, the ability to drive vehicles or work with other automated systems may be impaired.
Amlodipine
Amlodipine may have minor or moderate influence on the ability to drive and use machines. If patients taking amlodipine experience adverse reactions such as dizziness, headache, fatigue or nausea, their reactions may be reduced. Caution is recommended, especially at the beginning of treatment.
Method of administration and doses
The drug TENZOKARD is intended for oral use.
The recommended dose of the drug is 1 capsule per day, preferably in the morning before meals. The capsule is swallowed whole with water. The maximum daily dose is 1 capsule (10 mg/1.5 mg).
The use of a fixed combination is not intended for initiation of therapy.
If dosage changes are necessary, individual titration of each component of the combination should be performed.
Special patient groups
Patients with renal impairment (see sections "Contraindications" and "Special warnings and precautions for use")
In severe renal impairment (creatinine clearance
Elderly patients (see sections "Pharmacokinetics" and "Special warnings and precautions for use")
In elderly patients, the drug TENZOKARD should be used taking into account kidney function.
Patients with impaired liver function (see sections "Contraindications" and "Special instructions for use")
In severe hepatic impairment, treatment with the drug is contraindicated. For patients with mild to moderate hepatic impairment, dosage recommendations for amlodipine have not been established, therefore the dose should be selected with caution and treatment should be initiated at the lowest dose (see sections "Pharmacokinetics" and "Special warnings and precautions for use").
Children.
The safety and efficacy of the combination of amlodipine/indapamide in children have not been studied. Data are not available.
Overdose
There is no information on overdose with the amlodipine/indapamide combination in humans.
Overdose when using indapamide
Symptoms
When using indapamide in doses up to 40 mg, which is 27 times higher than the therapeutic dose, no toxic effects were detected. Symptoms of acute poisoning are mainly manifested in the form of disturbances of water and electrolyte balance (hyponatremia, hypokalemia). Clinically, nausea, vomiting, arterial hypotension, convulsions, vertigo, drowsiness, confusion, polyuria or oliguria, which can lead to anuria (due to hypovolemia), are possible.
Treatment
First aid measures include rapid removal of the ingested substance by gastric lavage and/or administration of activated charcoal, followed by normalization of water and electrolyte balance in a hospital setting.
Amlodipine overdose
Data on deliberate human overdose are limited.
Symptoms
From the available data it can be assumed that the use of very high doses may be associated with excessive peripheral vasodilation and possible reflex tachycardia. Pronounced, possibly prolonged systemic hypotension, leading to shock with fatal outcome, has been reported.
Treatment
Clinically significant hypotension caused by amlodipine overdose requires active cardiovascular care, including frequent monitoring of cardiac and respiratory function, positioning the patient in a horizontal position with elevation of the lower extremities, and monitoring of BCC and urine output. Administration of a vasoconstrictor may be useful to restore vascular tone and blood pressure if not contraindicated. Intravenous calcium gluconate is likely to help reverse the effects of calcium channel blockade. Gastric lavage may be useful in some cases. Administration of activated charcoal within 2 hours of a 10 mg dose of amlodipine reduces the rate of absorption. Since amlodipine is highly protein bound, hemodialysis is unlikely to be effective.
Adverse reactions
During treatment with indapamide and amlodipine, adverse reactions have been reported with the following frequencies: very common (≥ 1/10); common (≥ 1/100, ≤ 1/100); rare (≥ 1/10,000, ≤ 1/1,000); very rare (≤ 1/10,000); frequency unknown (cannot be estimated from the available data).
Infections and infestations: rhinitis (uncommon – amlodipine).
3. Blood and lymphatic system disorders: leukopenia (very rare – indapamide, amlodipine), thrombocytopenia (very rare – indapamide, amlodipine), agranulocytosis (very rare – indapamide), aplastic anemia (very rare – indapamide), hemolytic anemia (very rare – indapamide).
3. Immune system disorders: hypersensitivity (very rare – amlodipine).
3 Metabolism and metabolism: hypokalemia (often - indapamide (during clinical studies hypokalemia (10% of patients had a potassium level in the blood plasma, in 4% of patients the potassium level in the blood plasma was 4-6 weeks of treatment; after 12 weeks of therapy the average decrease in potassium level in the blood plasma was 0.23 mmol/l (see section "Special instructions")), hyperglycemia (very rarely - amlodipine), hypercalcemia (very rarely - indapamide), hyponatremia with hypovolemia* (uncommon - indapamide); hypochloremia (rarely - indapamide); hypomagnesemia (rarely - indapamide).
Psychiatric: insomnia (uncommon – amlodipine), mood changes (including anxiety) (uncommon – amlodipine), depression (uncommon – amlodipine), confusion (rare – amlodipine).
3 nervous system: drowsiness (often - amlodipine (especially at the beginning of treatment)), dizziness (often - amlodipine (especially at the beginning of treatment)), headache (rarely - indapamide, often - amlodipine (especially at the beginning of treatment)), tremor (uncommon - amlodipine), dysgeusia (uncommon - amlodipine), syncope (frequency unknown - indapamide, uncommon - amlodipine), hypoesthesia (uncommon - amlodipine), paresthesia (rarely - indapamide, uncommon - amlodipine), hypertonia (very rarely - amlodipine), peripheral neuropathy (very rarely - amlodipine), extrapyramidal disorders (extrapyramidal syndrome) (frequency unknown - amlodipine), in case of liver failure, hepatic encephalopathy may occur (see sections "Contraindications" and "Special instructions for use") (frequency unknown - indapamide).
3. Visual organs: visual impairment (frequency unknown - indapamide, often - amlodipine), diplopia (often - amlodipine), myopia (frequency unknown - indapamide), acute angle-closure glaucoma (frequency unknown - indapamide), blurred vision (frequency unknown - indapamide), choroidal effusion (frequency unknown - indapamide).
3. Hearing and vestibular system: tinnitus (infrequently - amlodipine), vertigo (rarely - indapamide).
Cardiac disorders: palpitations (often - amlodipine), myocardial infarction (very rarely - amlodipine), arrhythmia (including bradycardia, ventricular tachycardia, atrial fibrillation) (very rarely - indapamide, infrequently - amlodipine), paroxysmal ventricular tachycardia of the "pirouette" type (potentially fatal) (frequency unknown - indapamide (see sections "Special instructions" and "Interaction with other medicinal products and other types of interactions")).
3 vascular side: flushing (often – amlodipine), arterial hypotension (very rarely – indapamide, infrequently – amlodipine), vasculitis (very rarely – amlodipine).
Respiratory, thoracic and mediastinal disorders: dyspnea (often – amlodipine), cough (uncommon – amlodipine).
Gastrointestinal: abdominal pain (often - amlodipine), nausea (rarely - indapamide, often - amlodipine), vomiting (uncommon - indapamide, amlodipine), dyspepsia (often - amlodipine), change in the rhythm of defecation (often - amlodipine), dry mouth (rarely - indapamide, infrequently - amlodipine), pancreatitis (very rarely - indapamide)
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.