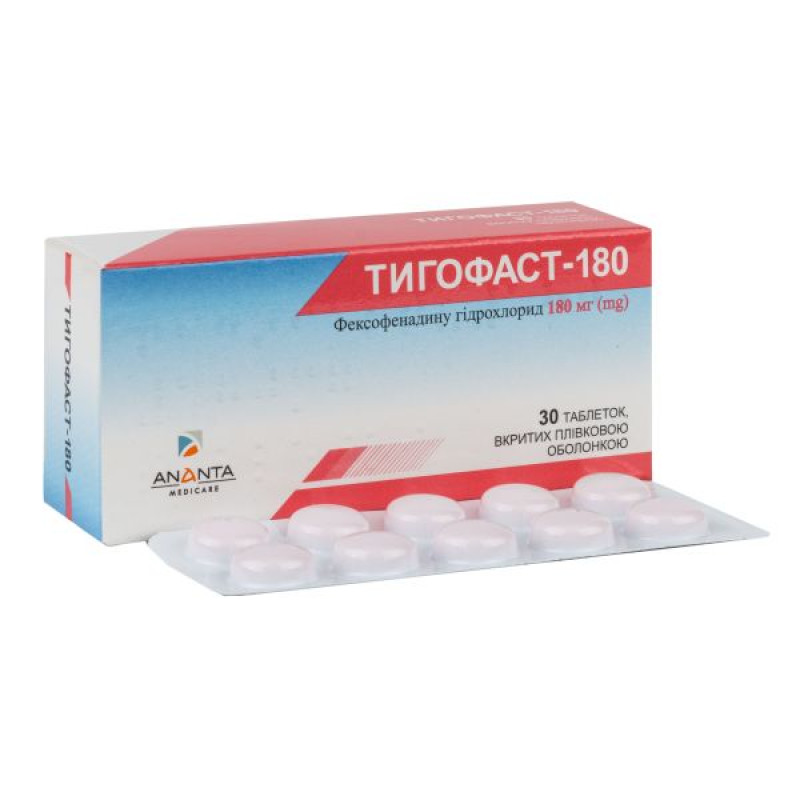
Tigofast-180 film-coated tablets 180 mg blister No. 30
Instructions for use Tigofast-180 film-coated tablets 180 mg blister No. 30
Composition
active ingredient: fexofenadine hydrochloride;
1 film-coated tablet contains fexofenadine hydrochloride 180 mg;
excipients:
180 mg tablets: microcrystalline cellulose, corn starch, sodium starch glycolate (type A), colloidal anhydrous silicon dioxide, Insta Coat Sunset Yellow film coating (ethylcellulose, hydroxypropylmethylcellulose, Sunset Yellow FCF (E 110)), talc.
Dosage form
Film-coated tablets.
Main physicochemical properties:
180 mg tablets: film-coated tablets, round, biconvex, orange in color.
Pharmacotherapeutic group
Antihistamines for systemic use. ATX code R06A X26.
Pharmacological properties
Pharmacodynamics
Fexofenadine hydrochloride is a non-sedating antihistamine from the group of specific H1 receptor antagonists. Fexofenadine is a pharmacologically active metabolite of terfenadine. Stabilizes mast cell membranes, prevents the release of histamine. Eliminates allergy symptoms: sneezing, rhinorrhea, itching, redness of the eyes and tearing. Does not have a sedative effect.
The antihistamine effect of fexofenadine hydrochloride administered once and twice daily was evident within 1 hour, peaked at 6 hours, and lasted for 24 hours. No signs of tolerance were observed even after 28 days of administration. Clinical efficacy was observed after single oral doses of 10 to 130 mg. A dose of 120 mg was sufficient to provide 24-hour efficacy.
Even at plasma concentrations 32 times higher than therapeutic concentrations, fexofenadine had no effect on slow potassium tubules of the human heart.
Fexofenadine hydrochloride (5–10 mg/kg orally) stops antigen-induced bronchospasm in sensitized animals and, at concentrations above therapeutic (10–100 micromolar), causes histamine release from peritoneal mast cells.
Pharmacokinetics
Fexofenadine hydrochloride is rapidly absorbed after oral administration. Maximum concentration is reached after approximately 1–3 hours. At a daily dose of 120 mg, the average maximum concentration is ≈ 427 ng/ml. At a daily dose of 180 mg, the average maximum concentration is ≈ 494 ng/ml.
60–70% of fexofenadine binds to plasma proteins. The active substance does not penetrate the blood-brain barrier.
Fexofenadine is almost not metabolized (both in the liver and outside it): only fexofenadine has been detected in significant quantities in the urine and feces of humans and animals.
Fexofenadine is eliminated from plasma with a biexponential decline and a terminal half-life of 11 to 15 hours after multiple dosing. Single and multiple dose kinetics are linear at oral doses up to 120 mg twice daily. In the saturation phase, doses up to 240 mg twice daily caused an increase in AUC that was slightly more than proportional (8.8%). This indicates that fexofenadine pharmacokinetics are nearly linear at daily doses of 40–240 mg.
Most of the dose is excreted in the bile, with up to 10% excreted unchanged in the urine.
Mutagenic and carcinogenic properties.
Various in vitro and in vivo mutagenicity tests did not reveal any mutagenic properties of fexofenadine hydrochloride.
In a carcinogenicity study, fexofenadine exposure was determined (based on plasma AUC) following administration of terfenadine in secondary pharmacokinetic studies. No evidence of carcinogenicity was observed when terfenadine was administered to rats and mice (up to 150 mg/kg body weight per day).
Indication
Symptomatic treatment of chronic idiopathic urticaria in adults and children aged 12 years and over.
Contraindication
Hypersensitivity to the components of the drug, age up to 12 years.
Interaction with other medicinal products and other types of interactions
Tigofast is not biotransformed in the liver and therefore does not interact with other drugs that are metabolized by microsomal liver enzymes. When Tigofast is taken simultaneously with erythromycin or ketoconazole, the concentration of Tigofast in the blood plasma increases by 2-3 times, which is due to increased absorption in the digestive tract and reduced elimination with bile. These changes are not accompanied by a change in the QT interval and do not cause an increase in the frequency of adverse reactions compared to the frequency of adverse reactions when each of these drugs is prescribed separately. No interaction of Tigofast and omeprazole was observed. When taking antacids containing aluminum or magnesium 15 minutes before taking Tigofast, its bioavailability decreases due to binding in the digestive tract. It is recommended to leave a 2-hour interval between taking Tigofast and antacids containing aluminum or magnesium hydroxide.
Application features
Patients with a history of or current cardiovascular disease should be aware that antihistamines may contribute to the development of side effects such as tachycardia and palpitations (see section "Adverse Reactions").
Ability to influence reaction speed when driving vehicles or other mechanisms
Based on the pharmacodynamic profile and known side effects, it can be concluded that taking Tigofast does not affect the ability to drive vehicles and perform work that requires concentration of attention. When conducting objective studies, it was found that Tigofast does not have a significant effect on the functions of the central nervous system (CNS). However, it is recommended to assess the individual response to the drug before starting to drive vehicles or perform work that requires concentration of attention.
Use during pregnancy or breastfeeding
Pregnancy: There are no adequate data from use in pregnant women. Limited animal studies do not indicate direct or indirect harmful effects with respect to pregnancy, embryonal/fetal development, parturition or postnatal development. Fexofenadine hydrochloride should not be used during pregnancy unless clearly necessary, when the expected benefit to the mother outweighs the potential risk to the fetus.
Breastfeeding. Since fexofenadine passes into breast milk, the drug should not be used during breastfeeding.
Method of administration and doses
Adults and children over 12 years of age should be prescribed Tigofast for seasonal allergic rhinitis - 120 mg once a day, for chronic idiopathic urticaria - 180 mg once a day. Take orally before meals, with water. The duration of treatment should be determined individually, depending on the severity of the disease.
Children under 12 years old
No studies have been conducted to study the efficacy and tolerability of Tigofast-120 or Tigofast-180 in children under 12 years of age.
Individual populations
According to the results of studies involving patients from certain risk groups (elderly patients, patients with impaired renal or hepatic function), dose adjustment is not required for such patients.
The duration of treatment depends on the course of the disease and is determined by the doctor individually.
Children
In this dosage, the drug should not be used in children under 12 years of age.
Overdose
Most reports of overdose with fexofenadine hydrochloride are not informative enough. For example, dizziness, drowsiness, and dry mouth have been reported.
In case of overdose, the usual measures should be taken to remove unabsorbed active substances. Symptomatic and supportive therapy is recommended. Removal of fexofenadine hydrochloride from the blood by hemodialysis is ineffective.
Adverse reactions
From the nervous system: headache, drowsiness, dizziness.
Gastrointestinal: nausea, diarrhea, epigastric cramps.
General disorders and administration site conditions: feeling of increased fatigue.
Immune system: hypersensitivity reactions, including angioedema, chest tightness, shortness of breath, flushing and systemic anaphylactic reactions, dyspnea, flushing.
Psychiatric: insomnia, increased irritability and sleep disturbances or unusual dreams (paroniria).
Cardiac: tachycardia, palpitations.
Skin and subcutaneous tissue disorders: rash, exanthema, urticaria, itching.
The medicine contains the dye "Yellow Sunset FCF" (E 110), which may cause allergic reactions.
Expiration date
2 years.
Do not use the drug after the expiration date.
Storage conditions
Keep out of reach of children. Store in original packaging at a temperature not exceeding 25 ºС.
Packaging
10 tablets in a blister, 3 blisters in a pack.
Vacation category
Without a prescription.
Producer
Artura Pharmaceuticals Pvt. Ltd.
Location of the manufacturer and its business address
1505 Portia Road, Sri City SEZ, Setyavedu Mandal, Chittoor District – 517 588, Andhra Pradesh, India.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.