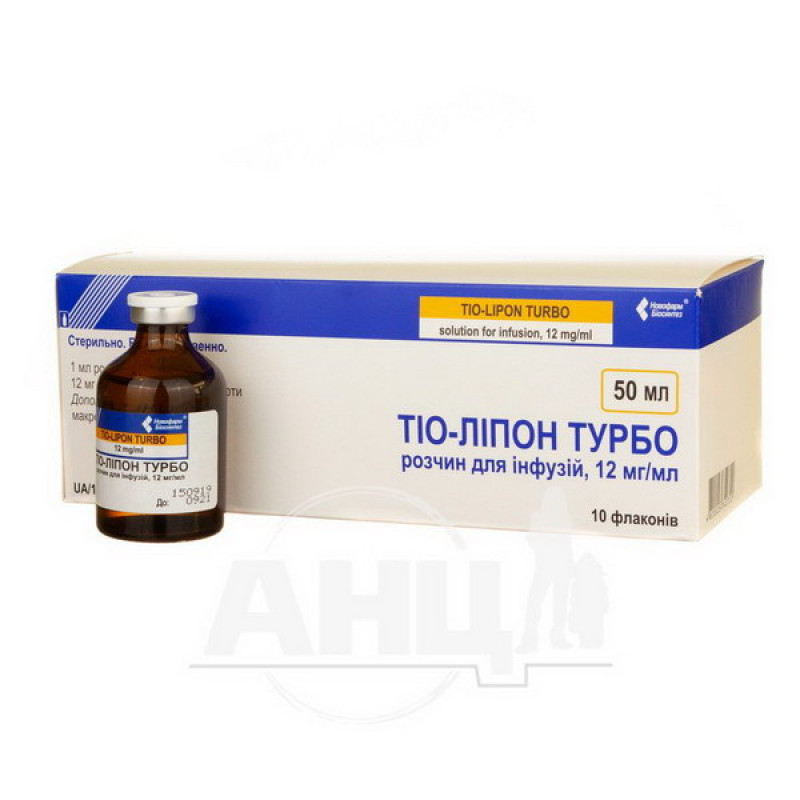
Tio-Lipon Turbo solution for infusions 12 mg/ml bottle 50 ml No. 10
Instructions for use Tio-Lipon Turbo solution for infusion 12 mg/ml bottle 50 ml No. 10
Composition
active ingredient: thioctic acid;
1 ml of solution contains 12 mg of thioctic (a-lipoic) acid;
Excipients: meglumine, macrogol 300, water for injections.
Dosage form
Solution for infusion.
Main physicochemical properties: yellowish or greenish-yellow solution.
Pharmacotherapeutic group
. Drugs affecting the digestive system and metabolic processes. ATX code A16A X01.
Pharmacological properties
Pharmacodynamics
Thioctic (a-lipoic) acid is a substance that is synthesized in the body and acts as a coenzyme in the oxidative decarboxylation of α-keto acids; it plays an important role in the process of energy formation in the cell. It helps to reduce blood sugar levels and increase the amount of glycogen in the liver. Deficiency or disruption of thioctic (a-lipoic) acid metabolism due to intoxication or excessive accumulation of certain decay products (for example, ketone bodies) leads to disruption of aerobic glycolysis. Thioctic (a-lipoic) acid can exist in two physiologically active forms (oxidized and reduced), which have antitoxic and antioxidant effects. Thioctic (a-lipoic) acid affects cholesterol metabolism, participates in the regulation of lipid and carbohydrate metabolism, and improves liver function (due to hepatoprotective, antioxidant, and detoxification effects). Thioctic (a-lipoic) acid is similar in pharmacological properties to B vitamins.
Pharmacokinetics
Thioctic (α-lipoic) acid undergoes significant changes during the first pass through the liver. There are significant interindividual fluctuations in the systemic availability of thioctic (α-lipoic) acid. It is excreted by the kidneys mainly in the form of metabolites. The formation of metabolites occurs as a result of side chain oxidation and conjugation. The half-life of thioctic (α-lipoic) acid from blood serum is 10-20 minutes.
Indication
Sensory impairment in diabetic polyneuropathy.
Contraindication
Hypersensitivity to thioctic (α-lipoic) acid or to other components of the drug. Heart and respiratory failure, acute phase of myocardial infarction, acute cerebrovascular accident, dehydration, chronic alcoholism and other conditions that can lead to lactic acidosis.
Interaction with other medicinal products and other types of interactions
Thioctic (a-lipoic) acid interacts with metal ion complexes (for example, with cisplatin), so the drug can reduce the effect of cisplatin. Thioctic (a-lipoic) acid forms poorly soluble complex compounds with sugar molecules. Thus, a solution of thioctic (a-lipoic) acid is incompatible with glucose solution, Ringer's solution and with solutions that can react with compounds containing SH-groups or disulfide bonds.
Do not use with drugs containing metals (e.g. iron, magnesium).
Thioctic (α-lipoic) acid may enhance the blood sugar-lowering effect of insulin and/or other antidiabetic agents, therefore, especially at the beginning of treatment with thioctic (α-lipoic) acid, regular monitoring of blood sugar levels is indicated. To prevent the appearance of symptoms of hypoglycemia, in individual cases, a reduction in the dose of insulin and/or oral antidiabetic agent may be required.
Ethanol reduces the therapeutic efficacy of thioctic (a-lipoic) acid.
Application features
When using the drug, light-protective black bags should be used, which are placed on top of the vial when administering the drug intravenously.
Do not use any remaining medicine.
The main factor in effective treatment of diabetic polyneuropathy is optimal correction of the patient's blood sugar level.
With parenteral administration of the drug there is a risk of allergic reactions, including anaphylactic shock, so patients should be monitored for such reactions. In case of symptoms such as itching, nausea, malaise, the drug should be immediately discontinued and the necessary therapeutic measures should be taken.
In isolated patients with decompensated or inadequately controlled diabetes and worsening general health, severe anaphylactic reactions associated with the use of the drug may develop.
In the treatment of patients with diabetes mellitus, frequent monitoring of blood glucose is necessary, especially at the beginning of treatment. In some cases, it is necessary to adjust the doses of antidiabetic drugs to prevent hypoglycemia.
During the treatment of polyneuropathy, due to regeneration processes, a short-term increase in sensitivity is possible, accompanied by paresthesia with a feeling of "crawling ants."
Warning: Regular alcohol consumption is a significant risk factor for the development and progression of the clinical picture of neuropathy and may negatively affect the treatment process with the drug. Therefore, patients with diabetic polyneuropathy are usually advised to abstain from alcohol whenever possible. Limiting alcohol consumption also applies to breaks between courses of treatment.
Use during pregnancy or breastfeeding
There is insufficient experience with the use of the drug during pregnancy or breastfeeding, so it should not be prescribed during these periods.
Ability to influence reaction speed when driving vehicles or other mechanisms
During the use of the drug, caution should be exercised when driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Method of administration and doses
The drug is administered directly from the vial (i.e. without solvent) as an intravenous drip infusion to adults at a dose of 600 mg per day (contents of 1 vial) for at least 30 minutes.
Due to the fact that thioctic (a-lipoic) acid is sensitive to light, the vials should be stored in cardboard packaging until their immediate use.
At the beginning of the treatment course, the drug is administered intravenously. The course of treatment is 2−4 weeks.
For further therapy, use oral forms of thioctic (a-lipoic) acid preparations at a dose of 300−600 mg per day.
Children
The efficacy and safety of the drug in children have not been established, so it should not be prescribed to this age group of patients.
Overdose
Symptoms: nausea, vomiting and headache are possible. When using very high doses of 10 to 40 g of thioctic (a-lipoic) acid in combination with alcohol, severe intoxication is observed, which can be fatal. The clinical picture of poisoning is initially manifested by psychomotor agitation or impaired consciousness and subsequently proceeds with attacks of generalized convulsions and the development of lactic acidosis. The consequences of intoxication may be hypoglycemia, shock, rhabdomyolysis, hemolysis, acute necrosis of skeletal muscles, disseminated intravascular coagulation, bone marrow suppression and multiorgan failure.
Treatment. If significant intoxication is suspected (> 80 mg/kg body weight of thioctic (a-lipoic) acid), immediate hospitalization and implementation of generally accepted measures (e.g., induction of vomiting, gastric lavage, use of activated charcoal) are indicated. Treatment of generalized convulsive attacks, lactic acidosis and other consequences of intoxications that threaten the patient's life should be guided by modern principles of intensive care and carried out symptomatically. To date, there is no data on the feasibility of using hemodialysis, hemoperfusion or hemofiltration methods within the framework of forced removal of thioctic (a-lipoic) acid.
Adverse reactions
From the side of the central nervous system: in some cases, changes or disturbances in taste sensations, headache, hot flashes, increased sweating, difficulty breathing, increased intracranial pressure, dizziness, convulsions, visual disturbances and double vision have been observed. In most cases, all of these manifestations resolve on their own.
On the part of the digestive tract: in some cases, with rapid intravenous administration of the drug, nausea, vomiting, diarrhea, abdominal pain were observed, which resolved on their own.
On the part of the blood system: in isolated cases, petechial hemorrhages in the mucous membranes/skin, platelet dysfunction, hypocoagulation, hemorrhagic rashes (purpura), thrombophlebitis were observed.
Metabolic disorders: due to improved glucose absorption, in some cases blood sugar levels may decrease, which may cause symptoms similar to hypoglycemia, such as dizziness, increased sweating, headache, and visual disturbances.
On the part of the immune system: in isolated cases, skin rashes, urticaria, itching, eczema, as well as systemic reactions up to the development of anaphylactic shock have been observed.
Cardiovascular system: with rapid intravenous administration, pain in the heart area and tachycardia may occur, which resolve spontaneously.
Other: In isolated cases, injection site reactions and weakness have been reported.
Expiration date
2 years.
Storage conditions
Store in original packaging at a temperature not exceeding 25 ° C. Keep out of the reach of children.
Incompatibility
Thioctic (α-lipoic) acid solution is incompatible with glucose solution, Ringer's solution and with solutions that may react with compounds containing SH groups or disulfide bonds. It cannot be used together with drugs containing metal compounds (for example, iron, magnesium preparations).
Packaging
50 ml in bottles; 1 bottle or 10 bottles in a cardboard pack.
Vacation category
According to the recipe.
Producer
Limited Liability Company "Novopharm-Biosyntez".
Ukraine, 11700, Zhytomyr region, Novograd-Volynskyi, Zhytomyrska st., 38.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.