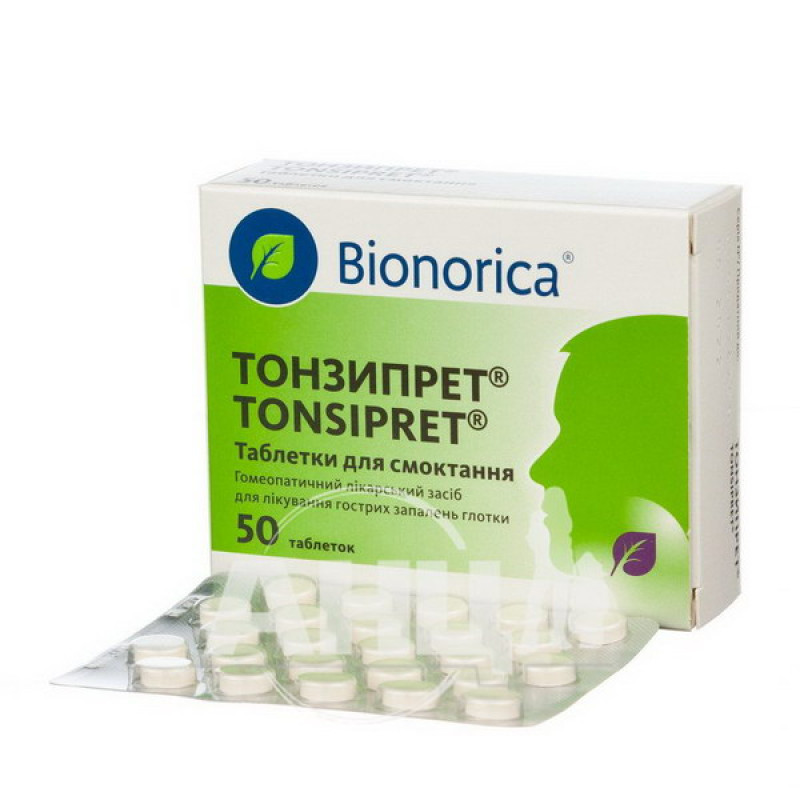
Tonzipret lozenges No. 50
Instructions for Tonzipret lozenges No. 50
Composition
active ingredients: 1 tablet contains:
| Phytolacca americana Ø (American Phytolacca Ø) | 50 mg, |
| Guaiacum D3 (guaiac tree D3) | 75 mg, |
| Capsicum annuum D3 (Capsicum D3) | 75 mg; |
excipients: potato starch, lactose monohydrate, magnesium stearate.
Dosage form
Lozenges.
Main physicochemical properties: round, light beige, flat-cylindrical tablets with a bevel and a break line on one side.
Pharmacotherapeutic group
A complex homeopathic preparation.
Pharmacological properties
The composition of the complex homeopathic preparation includes medicinal plants containing saponins, flavonoids, essential oils. Capsicum contains hot substances capsaicinoids, the main component of which is capsaicin. The composition of medicinal plants, which are components of the preparation, determines the anti-inflammatory and analgesic effect of the drug. American laconus, which is part of the preparation, also has an immunostimulating effect.
Indication
Acute and chronic inflammation of the pharynx, throat and tonsils (tonsillitis, laryngitis, pharyngitis).
Contraindication
Individual hypersensitivity to the active substances or to other components of the medicinal product. Pregnancy or breastfeeding.
Interaction with other medicinal products and other types of interactions
There is currently no information about any interactions with other drugs.
The effectiveness of homeopathic medicines can be negatively affected by an unhealthy lifestyle and the use of stimulants.
You should consult a doctor before using it simultaneously with other medications.
Application features
If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicine. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine.
If symptoms of the disease persist for a long time or if unclear or new complaints appear, as well as if the temperature persists for more than 3 days or rises above 39 °C, you should consult a doctor.
Note for diabetics: 1 tablet contains an average of 0.02 bread units.
Use during pregnancy or breastfeeding
Do not use the medicine during pregnancy or breastfeeding (see section "Contraindications").
Ability to influence reaction speed when driving vehicles or other mechanisms
There are no special precautions.
Method of administration and doses
Always use Tonzipret® as directed. If necessary, consult your doctor before using the drug.
Unless otherwise directed by your doctor, the drug should be taken as follows:
adults and children over 12 years of age for acute illnesses - 1 tablet every 30–60 minutes, but no more than 6 tablets per day;
children from 1 to 12 years old for acute illnesses - half a tablet every 30–60 minutes, but no more than 6 tablets per day.
If symptoms last more than a week, after consulting a doctor, the drug should be taken as follows:
adults and children over 12 years old - 1 tablet 1–3 times a day;
children from 1 to 12 years old - half a tablet 1–3 times a day.
After symptoms have subsided, the frequency of administration should be reduced.
The tablet should be sucked or allowed to dissolve slowly in the mouth. Children under 3 years of age are recommended to use Tonzipret® in the form of drops. For young children, it is possible to make it easier to swallow the tablet by dissolving the halves of the tablet in water.
Do not take a double dose if you missed the previous dose of Tonzipret®.
Stopping taking Tonzipret® tablets is usually safe.
If you have any further questions about the use of this medicine, ask your doctor.
Homeopathic medicines should not be taken for a long period of time without consulting a doctor.
Children
Due to lack of experience with the drug Tonzipret® should not be used in children under 1 year of age.
Overdose
When taking large doses of the drug, significantly exceeding the recommended ones (100 or 200 tablets), patients with hereditary lactose intolerance may experience stomach upset or diarrhea. It is necessary to immediately rinse the stomach and seek medical help.
Side effects
Nausea and stomach upset may occur, as well as allergic reactions, including skin rashes, erythema, and urticaria.
When using homeopathic medicines, temporary exacerbation of the symptoms of the disease (primary exacerbation) is possible. In this case, as well as in the event of any adverse reactions, you should stop using the medicine and consult a doctor.
Expiration date
4 years.
Do not use after the expiry date stated on the packaging.
Storage conditions
Store in the original packaging at a temperature not exceeding 30 °C, out of the reach of children.
Packaging
25 tablets in a blister;
2 blisters No. 50 (25×2) or 4 blisters No. 100 (25×4) in a cardboard box.
Vacation category
Without a prescription.
Manufacturer/Applicant
Bionorica SE.
Location of the manufacturer and its business address/location of the applicant and/or the applicant's representative.
Kerchensteinerstrasse, 11-15, 92318 Neumarkt, Germany.
Contact details of the manufacturer's representative in Ukraine, LLC "Bionorika":
phone: 044 521 86 00; fax: 044 521 86 01, info@bionorica.ua
Producer
Bionorica SE.
Location of the manufacturer and its business address/location of the applicant and/or the applicant's representative.
Kerchensteinerstrasse, 11-15, 92318 Neumarkt, Germany.
Contact details of the manufacturer's representative in Ukraine, LLC "Bionorika":
phone: 044 521 86 00; fax: 044 521 86 01, info@bionorica.ua
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.