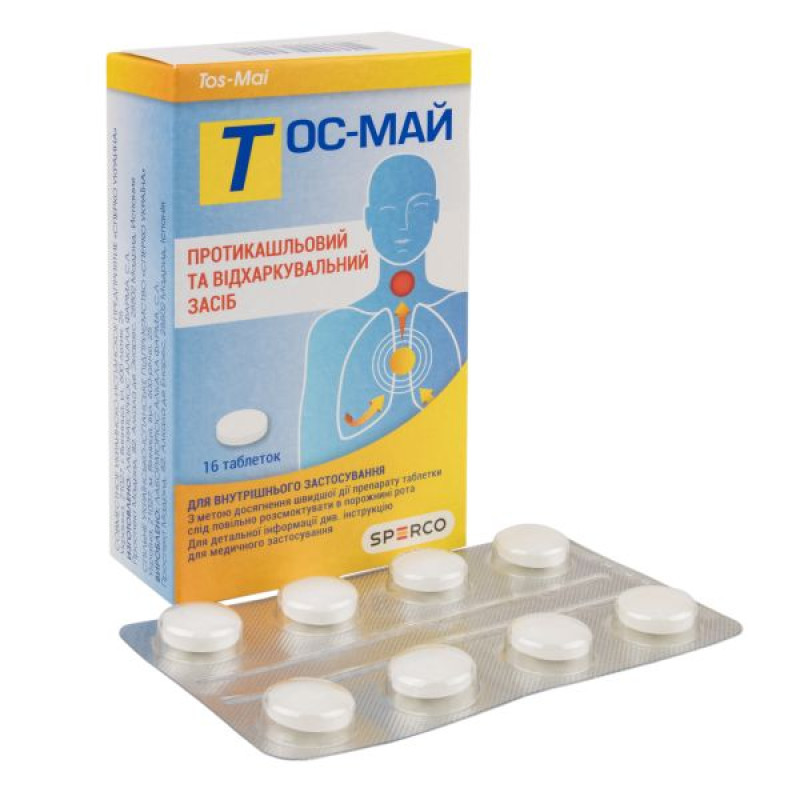
Tos-mai tablets blister pack No. 16
Instructions for Tos-May tablets blister No. 16
Composition
active ingredients: dextromethorphan hydrobromide, benzocaine, potassium guaiacolsulfonate, sodium benzoate;
1 tablet contains 2 mg of dextromethorphan hydrobromide, 0.2 mg of benzocaine, 15 mg of sodium benzoate, 35 mg of potassium guaiacolsulfonate;
excipients: mannitol (E 421), povidone, talc, magnesium stearate, anise essence, menthol, sodium saccharin.
Dosage form
Pills.
Main physicochemical properties: White tablets with inclusions, 13 mm in diameter.
Pharmacotherapeutic group
Antitussives and expectorants. ATX code R05F B.
Pharmacological properties
Pharmacodynamics. A combined drug that has antitussive, mucolytic, expectorant, and local anesthetic effects, thereby reducing cough, pain, and a feeling of sore throat. The effect of the drug is due to the pharmacological properties of its components.
Dextromethorphan is an antitussive, is the D-stereoisomer of levorphanol. By its mechanism of action, dextromethorphan is an antagonist of NMDA receptors in the medulla oblongata, affects the central part of the cough reflex, as a result of which dry unproductive cough associated with irritation of the mucous membrane of the respiratory tract in colds is reduced. The effect of dextromethorphan on the peripheral part of the cough reflex by suppressing impulses coming from the mucous membrane of the upper respiratory tract has also been noted. In terms of the severity of its antitussive action, dextromethorphan is close to codeine, but, unlike it, it does not cause addiction, does not suppress the respiratory center and the activity of the ciliated epithelium of the respiratory tract, and is devoid of analgesic action.
Benzocaine is a highly active surface local anesthetic, a derivative of para-aminobenzoic acid. It does not have a resorptive effect. The mechanism of action is associated with a decrease in the ionic permeability of nerve endings. As part of combined antitussive drugs, it reduces pain and a feeling of tickling in the throat.
Potassium guaiacol sulfonate belongs to expectorants. It helps reduce the viscosity of bronchial mucus, depolymerizes mucopolysaccharides and increases the activity of cilia of the ciliated epithelium of the respiratory tract. It reduces the surface tension and adhesive properties of sputum, reduces its viscosity and facilitates evacuation from the respiratory tract, transforming an unproductive cough into a productive one. It has a weak antiseptic and anesthetic effect.
Sodium benzoate belongs to the direct-acting expectorants. When taken internally, it increases the secretion of bronchial glands, thins phlegm and facilitates its evacuation from the respiratory tract. It has weak antibacterial and antifungal properties.
Pharmacokinetics. After oral administration, dextromethorphan is rapidly absorbed in the gastrointestinal tract and has high bioavailability. The antitussive effect develops 15-30 minutes after administration and lasts 5-6 hours. The maximum concentration in blood plasma is reached after 2 hours. It is actively metabolized in the liver by N- and O-demethylation. The half-life is 6.5 hours. It is excreted mainly by the kidneys in the form of metabolites, and only a small amount is excreted unchanged.
Potassium guaiacol sulfonate is rapidly absorbed from the gastrointestinal tract. The maximum concentration in the blood is reached after 1-2 hours. Therapeutic concentration is maintained for 6 hours. The half-life is 1-2 hours. It is excreted with sputum and excreted by the kidneys in the form of metabolites and in an unchanged state.
Indication
Symptomatic treatment of dry irritating cough in acute respiratory viral infections and infectious and inflammatory diseases of the upper and lower respiratory tract.
When preparing patients for bronchoscopy.
Contraindication
Hypersensitivity to dextromethorphan, amide anesthetics and other components of the drug. Respiratory failure, bronchial asthma, pulmonary emphysema.
Interaction with other medicinal products and other types of interactions
The simultaneous use of the drug with antidepressants - monoamine oxidase inhibitors (MAO), furazolidone, procarbazine should be avoided due to the increased risk of CNS excitation, development of arterial hypertension and hyperthermia. CNS depressants and alcohol, when used together, enhance the sedative effect of the drug. Quinidine slows down the metabolism of dextromethorphan in the liver, increasing its concentration in the blood.
Application features
Do not exceed the recommended doses of the drug, combine the use of the drug with alcoholic beverages, and also use the drug for chronic cough associated with bronchial asthma and emphysema. The drug should be prescribed with caution to persons with liver disease.
Use during pregnancy or breastfeeding
Teratogenic and embryotoxic effects of the drug have not been registered, but, given the lack of adequate clinical studies, Tos-May can be used during pregnancy only as prescribed by a doctor in cases where the potential benefit to the mother outweighs the potential risk to the fetus. Due to the lack of information on the use of the drug during breastfeeding, it should not be used by women during this period.
Ability to influence reaction speed when driving vehicles or other mechanisms
Some patients may experience drowsiness and decreased reaction time after using the drug, which should be taken into account when driving vehicles.
Method of administration and doses
Children over 12 years of age and adults usually take 1-2 tablets 4-6 times a day (maximum 16 tablets per day), children from 6 to 12 years of age - 1 tablet 4 times a day (maximum 8 tablets per day).
In order to achieve a faster effect of the drug, the tablets should be slowly dissolved in the oral cavity.
Children. Tos-Mai is prescribed for children aged 6 years and older.
Overdose
In case of a significant overdose, symptoms may occur due to:
dextromethorphan – drowsiness, dizziness, ataxia, irritability, hyperactivity, confusion, blurred vision, nystagmus, respiratory depression, nausea, vomiting; guaiacol sulfonate potassium – drowsiness, nausea, vomiting;
benzocaine – extremely rare methemoglobinemia.
There is no specific antidote, treatment is symptomatic.
Adverse reactions
On the part of the digestive tract: nausea, vomiting, abdominal pain, diarrhea.
From the side of the central nervous system: general weakness, drowsiness, headache.
On the part of the immune system: allergic reactions, including rash, itching, facial flushing, angioedema.
Expiration date
2 years. Do not use the drug after the expiration date indicated on the package.
Storage conditions
Store at a temperature not exceeding 25 0 C. Keep out of the reach of children.
Packaging
8 tablets in a blister of aluminum foil coated with PVDC and with a PVC/PVDC layer. 2 blisters with instructions in a cardboard pack.
Vacation category
Without a prescription.
Producer
LABORATORIOS ALKALA FARMA, S.L.
Location of the manufacturer and address of its place of business.
Avenida Madrid, 82, Alcala de Henares, 28802 Madrid, Spain.
Applicant.
Joint Ukrainian-Spanish enterprise "Sperco Ukraine".
Location of the applicant and address of the place of business.
21027, Ukraine, Vinnytsia, 600-anniversary st., 25.
Phone: + 38(0432)52-30-36. E-mail: trade@sperco.com.ua
www.sperco.ua
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.