Toujeo Solostar solution for injection 300 U/ml cartridge 1.5 ml mounted in a syringe pen without needle No. 1
SoloStar injection solution is used to treat diabetes mellitus in adults.
Composition
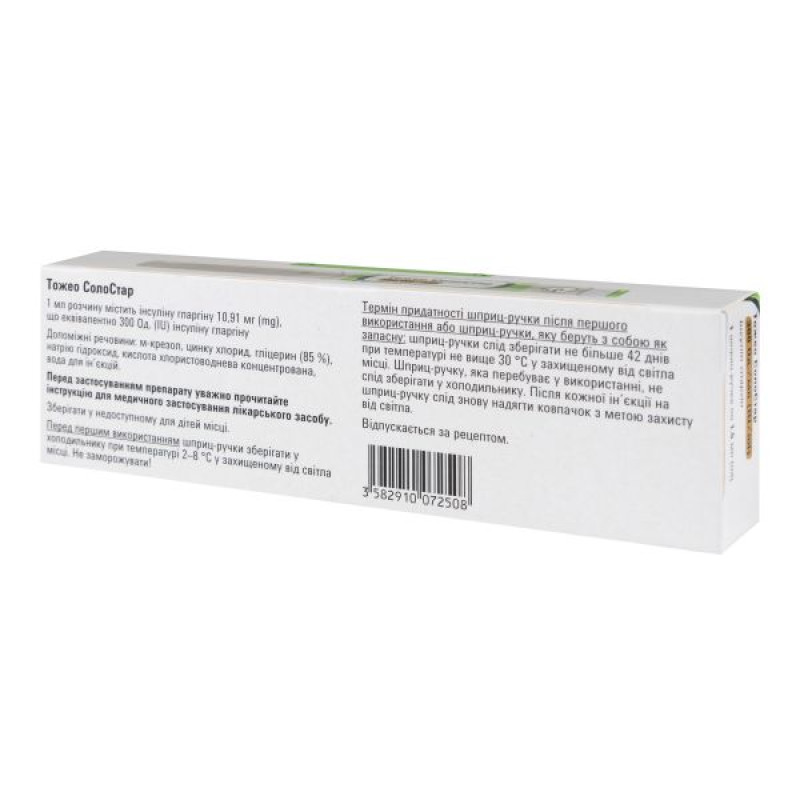
Active ingredient: insulin glargine (1 ml of solution contains 10.91 mg of insulin glargine, equivalent to 300 U of insulin glargine; 1 syringe pen contains 1.5 ml of solution for injection, equivalent to 450 U of insulin glargine).
Excipients: m-cresol, zinc chloride, glycerin (85%), sodium hydroxide, concentrated hydrochloric acid, water for injections.
Contraindication
Hypersensitivity to the active substance or to any excipient included in the preparation.
Method of application
The drug "also SoloStar" is a basal insulin preparation for administration once a day at any time of the day, but preferably at the same time every day.
The drug administration regimen (dose and time of administration) should be selected according to the patient's individual response to treatment.
In type 1 diabetes, the drug "also SoloStar" should be used in combination with short-acting/rapid-acting insulin to meet the need for insulin after meals.
For patients with type 2 diabetes, the drug "also SoloStar" can be used simultaneously with oral hypoglycemic drugs.
The potency of this medicine is expressed in units. These units are used exclusively for SoloStar and are different from the IU or units in which the potency of other insulin analogues is expressed.
Flexibility of input time
If necessary, patients may administer SoloStar up to 3 hours before or after their usual dosing time. Patients who forget to administer a dose are advised to check their blood sugar levels more frequently and then return to their usual once-daily dosing schedule. Patients should be cautioned against taking a double dose to make up for a missed dose.
Patients with type 1 diabetes. The drug "also SoloStar" requires individual dosage selection and should be used once a day together with short-acting insulin.
Patients with type 2 diabetes mellitus. The recommended daily dose is 0.2 units/kg with subsequent individual dose adjustment.
An introductory part of the instructions is provided, read the full instructions inside the package.
Application features
Pregnant women
The drug "Tozh SoloStar" can be prescribed during pregnancy if there is a need for this treatment.
It is not known whether insulin glargine is excreted in human milk. No metabolic effects are expected in the newborn/infant due to the passage of insulin glargine through breast milk during breastfeeding, as insulin glargine is a peptide that is broken down to amino acids in the human gastrointestinal tract. Breastfeeding women may require dose and dietary adjustments.
Studies in laboratory animals have not shown direct harmful effects on fertility.
Children
The safety and efficacy of the drug "also SoloStar" for the treatment of children under the age of 18 have not been established. Data on this issue are not available.
Drivers
The patient's ability to concentrate and react may be impaired due to hypoglycemia or hyperglycemia or, for example, due to visual disturbances. This may be dangerous in situations where these qualities are particularly important (e.g. when driving or operating machinery).
Patients should be advised to take the necessary precautions to avoid hypoglycemia while driving. This is especially important for those patients in whom the first signs of hypoglycemia are weak or absent, as well as for those patients who experience hypoglycemia frequently. Careful consideration should be given to whether to drive or operate machinery in this condition.
Overdose
Symptoms
An overdose of insulin can lead to severe and sometimes prolonged hypoglycemia, which can be life-threatening.
Treatment
Mild cases of hypoglycemia can usually be corrected by oral administration of carbohydrates. Adjustment of drug dosage, dietary changes, or physical activity may also be necessary.
More severe episodes of hypoglycemia, accompanied by coma, seizures or neurological disorders, require intramuscular / subcutaneous administration of glucagon or the introduction of a concentrated glucose solution. Since hypoglycemia can recur even after a clear improvement in the patient's clinical condition, prolonged carbohydrate intake and patient observation are necessary measures.
Side effects
Hypoglycemia is generally the most common adverse reaction observed with insulin therapy. It occurs when the dose of insulin administered exceeds the need for it.
Interaction
The drug "also SoloStar" cannot be mixed with any other insulin preparation or with other drugs or diluted with them. Mixing with other drugs or diluting this drug changes its time course of action, and mixing with other drugs causes the formation of a precipitate.
Storage conditions
Before first use, store the pen in a refrigerator at +2°C to +8°C, protected from light. Do not freeze.
Shelf life - 30 months.
Shelf life after first use of the pen or if the pen is carried as a spare: The pens should be stored for no more than 28 days at a temperature not exceeding 30°C, protected from light. The pen in use should not be stored in the refrigerator. The pen cap should be replaced after each injection to protect from light.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.