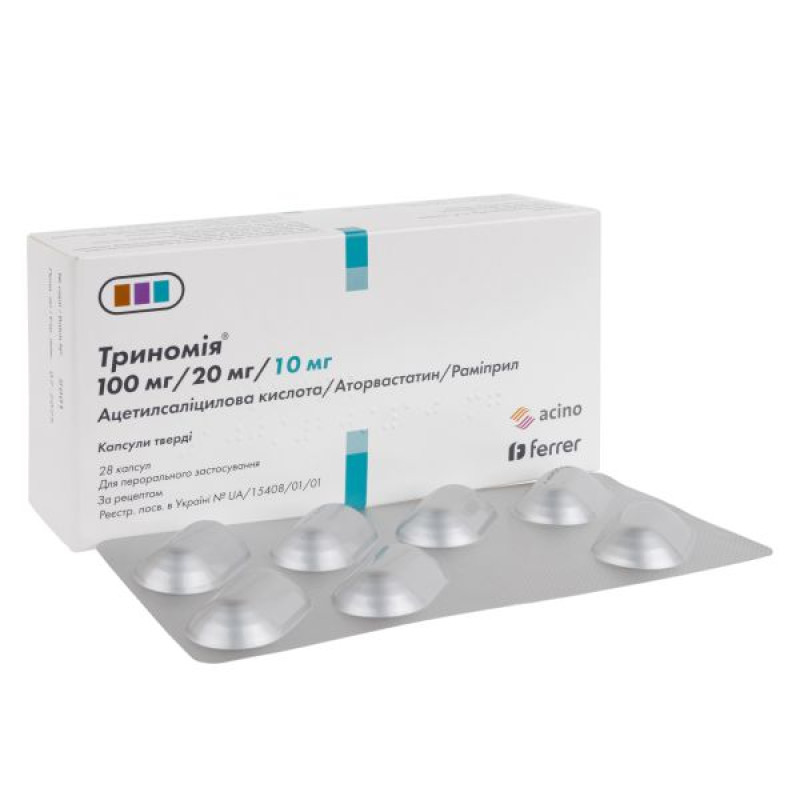
Trinomia hard capsules blister 100 mg + 20 mg + 10 mg No. 28
Instructions Trinomia hard capsules blister 100 mg + 20 mg + 10 mg No. 28
Composition
active ingredients:
1 capsule contains 100 mg of acetylsalicylic acid, 20 mg of atorvastatin (as atorvastatin calcium trihydrate) and 10 mg of ramipril;
excipients:
for acetylsalicylic acid tablets: microcrystalline cellulose; sodium starch glycolate (type A); talc; Opadry AMV white OY-B-28920;
for atorvastatin tablets: lactose monohydrate; pregelatinized starch 1500; calcium carbonate; hydroxypropylcellulose; polysorbate 80; crospovidone type A; colloidal anhydrous silica; magnesium stearate; Opadry green 06O21881;
for ramipril tablets: hypromellose 2910; microcrystalline cellulose, pregelatinized starch 1500; sodium stearyl fumarate; Opadry AMV yellow 80W32880;
hard capsule: gelatin; titanium dioxide (E 171); iron oxide, black (E 172); iron oxide, red (E 172); black ink.
Dosage form
The capsules are hard.
Main physicochemical properties: opaque, hard gelatin capsules, with a pale pink body and cap, with the inscription "AAR 100/20/10", containing two acetylsalicylic acid tablets, film-coated, white or almost white, engraved with "AS", two atorvastatin tablets, film-coated, greenish-brown, engraved with "AT" and one ramipril tablet, film-coated, light yellow, engraved with "R1".
Pharmacotherapeutic group
HMG-CoA reductase inhibitors, other combinations.
ATX code C10B X06.
Pharmacological properties
Pharmacodynamics
Acetylsalicylic acid. Acetylsalicylic acid irreversibly inhibits platelet aggregation. This effect on platelets is due to the acetylation of cyclooxygenase. This irreversibly inhibits the synthesis of thromboxane A2 (which stimulates platelet aggregation and has a vasoconstrictor effect) in platelets. This effect is permanent and usually lasts throughout the 8-day life span of platelets.
Acetylsalicylic acid also inhibits the synthesis of prostacyclin (a prostaglandin that inhibits platelet aggregation but has a vasodilating effect) in endothelial cells of blood vessels. This effect is temporary. After acetylsalicylic acid is eliminated from the blood, nucleated endothelial cells begin to synthesize prostacyclin again. As a result, a single low daily dose of acetylsalicylic acid (<100 mg/day) causes inhibition of thromboxane A2 in platelets without a significant effect on prostacyclin synthesis.
Acetylsalicylic acid belongs to the group of acid-forming nonsteroidal anti-inflammatory drugs with analgesic, antipyretic and anti-inflammatory properties. Their mechanism of action is the irreversible inhibition of cyclooxygenase enzymes, which are involved in the synthesis of prostaglandins. Higher doses of acetylsalicylic acid are used to treat mild to moderate pain, fever, and acute and chronic inflammatory diseases such as rheumatoid arthritis.
Experimental data have shown that ibuprofen may inhibit platelet aggregation when given concomitantly with low doses of acetylsalicylic acid. In a study comparing the effect of a single dose of ibuprofen 400 mg given 8 hours before or 30 minutes before taking 81 mg of acetylsalicylic acid (as an immediate-release tablet), a reduction in the effect of acetylsalicylic acid on thromboxane formation or platelet aggregation was observed. However, these data are limited, as there is uncertainty about the extrapolation of these data to clinical practice. Therefore, no relevant conclusion can be drawn regarding the regular use of ibuprofen, and there is no data regarding a relevant clinical effect that could be attributed to the occasional use of ibuprofen.
Atorvastatin. Atorvastatin is a selective competitive inhibitor of HMG-CoA reductase, an enzyme that determines the rate of conversion of 3-hydroxy-3-methyl-glutaryl-coenzyme A to mevalonate, a precursor of sterols, including cholesterol. Triglycerides and cholesterol in the liver are incorporated into very low density lipoprotein (VLDL) molecules, enter the blood plasma, and are transported to peripheral tissues. Low density lipoprotein (LDL) is formed from VLDL and is catabolized primarily by interaction with high-affinity LDL receptors (LDL receptors).
Atorvastatin reduces plasma cholesterol and serum lipoprotein concentrations by inhibiting HMG-CoA reductase and subsequently cholesterol biosynthesis in the liver, and also increases the number of hepatic LDL receptors on the cell surface, leading to increased uptake and catabolism of LDL.
Atorvastatin has been shown to reduce total cholesterol (30–46%), LDL-C (41–61%), apolipoprotein B (34–50%), and triglycerides (14–33%), while causing variable increases in HDL-C and apolipoprotein A1 in a dose-dependent study. These results are consistent with data in patients with heterozygous familial hypercholesterolemia, nonfamilial hypercholesterolemia, and mixed hyperlipidemia, including patients with noninsulin-dependent diabetes mellitus. Reductions in total cholesterol, LDL-C, and apolipoprotein B have been shown to reduce the risk of cardiovascular disease and mortality.
Ramipril. Ramiprilat, the active metabolite of the prodrug ramipril, inhibits the enzyme dipeptidyl carboxypeptidase I (synonyms: angiotensin-converting enzyme; kinase II). In plasma and tissues, this enzyme catalyzes the conversion of angiotensin I to the active vasoconstrictor angiotensin II and the degradation of the active vasodilator bradykinin. The reduction in the formation of angiotensin II and the inhibition of the degradation of bradykinin lead to vasodilation.
Since angiotensin II also stimulates the release of aldosterone, ramiprilat causes a decrease in aldosterone secretion. In black (Afro-Caribbean) hypertensive patients (usually low-renin hypertensive patients), the average response to monotherapy with an angiotensin-converting enzyme (ACE) inhibitor was lower than in patients of other races.
Hypotensive properties. The use of ramipril causes a marked decrease in peripheral arterial resistance. Usually renal plasma flow and glomerular filtration rate do not change. The use of ramipril in patients with arterial hypertension causes a decrease in blood pressure in the standing and supine positions, without a compensatory increase in heart rate. In most patients, after oral administration of a single dose, the antihypertensive effect is manifested after 1-2 hours, and the maximum effect is after 3-6 hours and usually lasts for 24 hours. With continued use of ramipril, the maximum antihypertensive effect is usually achieved after 3-4 weeks. It has been established that with long-term therapy, the antihypertensive effect is maintained for 2 years. Abrupt cessation of treatment with ramipril does not cause a rapid and excessive rebound increase in blood pressure.
Heart failure. As an adjunct to diuretic and cardiac glycoside therapy, ramipril has been shown to be effective in patients with heart failure functional classes II–IV according to the New York Heart Association classification. The drug has a beneficial effect on cardiac hemodynamics (reduction of left and right ventricular filling pressures, reduction of total peripheral vascular resistance, increase in cardiac output, and improvement of cardiac index). It also reduced neuroendocrine activation.
Pharmacokinetics
Acetylsalicylic acid. Acetylsalicylic acid is metabolized to its main active metabolite, salicylic acid, before, during and after absorption. The metabolites are excreted mainly by the kidneys. In addition to salicylic acid, the main metabolites of acetylsalicylic acid are the glycine conjugate of salicylic acid (salicylicuric acid), the glucuronide ester and ester of salicylic acid (salicylicphenol glucuronide and salicylacyl glucuronide), and gentisic acid, which is formed by the oxidation of salicylic acid and its glycine conjugate.
Absorption of acetylsalicylic acid after oral administration is rapid, complete and dependent on the galenic preparations. Hydrolysis of the acetyl residue of acetylsalicylic acid occurs to some extent during passage through the gastrointestinal mucosa. Maximum plasma concentrations are reached 10–20 minutes after administration (acetylsalicylic acid), or 0.3–2 hours (total salicylate).
The elimination kinetics of salicylic acid are largely dose-dependent, as the ability to metabolize salicylic acid is limited (the half-life ranges from 2 to 30 hours).
The half-life of acetylsalicylic acid is only a few minutes; the half-life of salicylic acid is 2 hours after a dose of 0.5 g of acetylsalicylic acid, 4 hours after 1 g, and increases to 20 hours after a single dose of 5 g.
Binding to plasma proteins in humans is concentration-dependent; values ranging from 49% to more than 70% (acetylsalicylic acid) and from 66% to 98% (salicylic acid) have been reported. Salicylic acid is found in cerebrospinal fluid and synovial fluid after administration of acetylsalicylic acid. Salicylic acid crosses the placenta and is excreted in breast milk.
Atorvastatin.
The absolute bioavailability of atorvastatin is approximately 12%, and the systemic availability of HMG-CoA reductase inhibitory activity is approximately 30%. The low systemic availability is due to presystemic clearance in the gastrointestinal mucosa and/or hepatic presystemic metabolism.
Distribution: The mean volume of distribution of atorvastatin is approximately 381 L. Plasma protein binding is ≥ 98%.
Biotransformation. Atorvastatin is metabolized by cytochrome P450 3A4 to ortho- and parahydroxylated derivatives and other beta-oxidation products. In addition to other metabolic pathways, these products are further glucuronidated. In vitro, ortho- and parahydroxylated metabolites cause inhibition of HMG-CoA reductase equivalent to its inhibition by atorvastatin. The inhibitory effect of the drug on HMG-CoA reductase is almost 70% determined by the activity of circulating metabolites.
Elimination: Atorvastatin is eliminated primarily in the bile following hepatic and/or extrahepatic metabolism. However, atorvastatin does not undergo significant hepatic recirculation. The mean elimination half-life of atorvastatin from human plasma is approximately 14 hours. The half-life of HMG-CoA reductase inhibitory activity is approximately 20–30 hours due to the presence of active metabolites.
Ramipril.
Absorption. After oral administration, ramipril is rapidly absorbed from the gastrointestinal tract: maximum plasma concentrations of ramipril are reached within 1 hour. Taking into account urinary excretion, the extent of absorption is at least 56% and is not significantly affected by the presence of food in the gastrointestinal tract. The bioavailability of the active metabolite ramiprilat after oral administration of 2.5 and 5 mg of ramipril is 45%.
Peak plasma concentrations of ramiprilat, the only active metabolite of ramipril, are reached 2-4 hours after administration of ramipril. After the use of usual doses of ramipril once daily, steady-state plasma concentrations of ramiprilat are reached after approximately 4 days of treatment.
Distribution: Plasma protein binding of ramipril is approximately 73% and that of ramiprilat is approximately 56%.
Metabolism: Ramipril is almost completely metabolized to ramiprilat and diketopiperazine ester, diketopiperazine acid, as well as the glucuronides of ramipril and ramiprilat.
Elimination. Elimination of metabolites occurs mainly by renal excretion. The decline in plasma concentrations of ramiprilat is multiphasic. Due to the strong saturable binding to ACE and the slow dissociation from the enzyme, ramiprilat exhibits a prolonged terminal elimination phase even at very low plasma concentrations.
The effective half-life of ramiprilat after repeated doses of 5–10 mg ramipril once daily is 13–17 hours and is longer at lower doses (1.25–2.5 mg). The difference is due to the saturable capacity of the enzyme to bind ramiprilat.
After a single oral dose, neither ramipril nor its metabolite were detected in breast milk. However, the effect of repeated doses is unknown.
Indication
Secondary prevention of cardiovascular complications in adult patients as replacement therapy when adequate control is provided during therapy with monocomponent agents in equivalent therapeutic doses.
Contraindication
Hypersensitivity to the active substances or other components of the drug, other salicylates, non-steroidal anti-inflammatory drugs (NSAIDs), other angiotensin-converting enzyme (ACE) inhibitors or tartrazine. Hypersensitivity to soy or peanut. History of asthma or other allergic reactions caused by the use of acetylsalicylic acid or other non-steroidal analgesics/anti-inflammatory drugs.
Acute peptic ulcers (see section "Special warnings and precautions for use").
Hemophilia and other blood clotting disorders (thrombocytopenia, hemorrhagic diathesis).
Severe renal and hepatic insufficiency (see section "Method of administration and dosage").
Contraindicated in patients undergoing hemodialysis (see section "Method of administration and dosage").
Severe heart failure, hypotension, hemodynamically unstable conditions.
Concomitant use with methotrexate at a dose of 15 mg/week or more (see section "Interaction with other medicinal products and other types of interactions").
Nasal polyps associated with asthma caused or exacerbated by the use of acetylsalicylic acid.
Liver disease or persistent unexplained elevation of serum transaminases greater than 3 times the upper limit of normal (see section 4.4).
Pregnancy and lactation. Contraindicated in women of childbearing potential not using effective contraception (see section "Use during pregnancy or lactation").
Concomitant use with tipranavir or ritonavir (due to the risk of rhabdomyolysis) (see sections “Special warnings and precautions for use” and “Interaction with other medicinal products and other types of interactions”).
Combination with methotrexate. History of angioedema (hereditary, idiopathic or induced by the use of ACE inhibitors or angiotensin II receptor antagonists).
Extracorporeal treatments that result in blood coming into contact with negatively charged surfaces (see section “Interaction with other medicinal products and other types of interactions”).
Severe bilateral renal artery stenosis or stenosis of the renal artery to a single functioning kidney. Ramipril should not be used in patients with hypotensive or hemodynamically unstable conditions. Children and adolescents under 18 years of age. Children under 16 years of age with fever, influenza or chickenpox are at risk of developing Reye's syndrome. Recent trauma, surgery.
Interaction with other medicinal products and other types of interactions
Acetylsalicylic acid: pharmacodynamic and pharmacokinetic interactions.
Anticoagulant and thrombolytic therapy: Acetylsalicylic acid increases the risk of bleeding when used before or during anticoagulant and thrombolytic therapy. Therefore, patients requiring anticoagulant and thrombolytic treatment should be monitored for signs of external or internal bleeding.
Other platelet aggregation inhibitors: Platelet aggregation inhibitors, such as ticlopidine and clopidogrel, may prolong blood clotting time.
Other nonsteroidal analgesics/anti-inflammatory and antirheumatic drugs. These drugs increase the risk of gastrointestinal bleeding and ulcers.
Systemic glucocorticoids (except hydrocortisone as replacement therapy for Addison's disease). Systemic glucocorticoids increase the risk of gastrointestinal ulcers and bleeding.
Alcohol: Alcohol increases the risk of gastrointestinal ulcers and bleeding.
Digoxin: NSAIDs increase the concentration of digoxin in the blood plasma. When used simultaneously with Trinomia or when it is discontinued, it is recommended to monitor the level of digoxin in the blood plasma.
Antidiabetic agents, including insulin. Concomitant use of Trinomia® with antidiabetic agents, including insulin, increases the hypoglycemic effect of these agents. Monitoring of blood glucose levels is recommended (see below, section “Ramipril: pharmacodynamic and pharmacokinetic interactions. Precautions for use”).
Methotrexate. Salicylates may displace methotrexate from plasma protein binding and reduce its renal clearance, leading to toxic plasma concentrations of methotrexate. Concomitant use with methotrexate at doses of 15 mg or more per week is contraindicated (see section 4.3). Renal function and blood counts should be monitored when methotrexate is administered at doses below 15 mg per week, especially at the beginning of treatment.
Valproic acid: Salicylates may displace valproic acid from plasma protein binding and reduce its metabolism by increasing its plasma concentrations.
Selective serotonin reuptake inhibitors (SSRIs): SSRIs increase the risk of bleeding, particularly gastrointestinal bleeding, due to a synergistic effect.
Diuretics: NSAIDs may cause acute renal failure, especially in patients with dehydration. In the case of concomitant use of Trinomia® and diuretics, it is recommended to monitor adequate hydration of patients.
Uricosuric agents. Concomitant use with Trinomia® reduces the effect of agents that promote the excretion of uric acid and increases plasma levels of acetylsalicylic acid by reducing its excretion.
ACE inhibitors: Although there have been reports that acetylsalicylic acid may reduce the beneficial effects of ACE inhibitors by reducing the synthesis of vasodilator prostaglandins, some studies have found that the negative interaction with ACE inhibitors occurs with high (i.e. ≥ 325 mg) rather than low (i.e. ≤ 100 mg) doses of acetylsalicylic acid.
Ibuprofen. There is no data on the possible interaction of acetylsalicylic acid and ibuprofen taken together for a long time, although some studies have shown a reduced effect on platelet aggregation.
Cyclosporine: NSAIDs may increase the nephrotoxicity of cyclosporine through effects mediated by renal prostaglandins. Close monitoring of renal function is recommended, especially in elderly patients.
Vancomycin: Acetylsalicylic acid increases the risk of vancomycin ototoxicity.
Interferon α. Acetylsalicylic acid reduces the activity of interferon α.
Lithium: NSAIDs reduce the excretion of lithium, increasing its plasma levels, which may reach toxic values. The combined use of lithium and NSAIDs is not recommended. If the use of such a combination is necessary, careful monitoring of plasma lithium concentrations should be carried out at the beginning, during dose adjustment and when treatment is discontinued.
Antacids: Antacids may increase the renal excretion of salicylates by alkalinizing the urine.
Zidovudine: Acetylsalicylic acid may increase plasma levels of zidovudine by competitively inhibiting the formation of its glucuronide or by directly inhibiting the metabolism of zidovudine by liver microsomal enzymes.
Phenytoin: Acetylsalicylic acid may increase plasma levels of phenytoin.
Laboratory tests: Acetylsalicylic acid may affect the results of the following tests:
Blood: increased levels (biological) of transaminases (alanine aminotransferase (ALT) and aspartate aminotransferase (AST)), alkaline phosphatase, ammonia, bilirubin, cholesterol, creatine kinase, digoxin, free thyroxine, lactate dehydrogenase (LDH), thyroxine-binding globulin, triglycerides, uric acid and valproic acid; increased levels (analytical interference) of glucose, paracetamol and total protein; decreased levels (biological) of free thyroxine, glucose, phenytoin, thyroid-stimulating hormone (TSH), thyrotropin-releasing hormone (TSH-RG), thyroxine, triglycerides, triiodothyronine, uric acid and creatinine clearance; decreased levels (analytical interference) of transaminases (ALT), albumin, alkaline phosphatase, cholesterol, creatine kinase, lactate dehydrogenase (LDH), and total protein.
Urine: decreased levels (biological) of estriol; decreased levels (analytical interference) of 5-hydroxyindoleacetic acid, 4-hydroxy-3-methoxymandelic acid, total estrogens and glucose.
Effect of concomitant medications on atorvastatin.
Atorvastatin is metabolized by cytochrome P450 3A4 (CYP3A4) and is a substrate for transport proteins, such as the hepatic uptake transporter OATP1B1. Concomitant use of medicinal products that are inhibitors of CYP3A4 or transport proteins may result in increased plasma concentrations of atorvastatin and an increased risk of myopathy. The risk is also increased when atorvastatin is co-administered with other medicinal products that can cause myopathy, such as fibric acid derivatives and ezetimibe (see section 4.4).
CYP3A4 inhibitors.
As noted, potent CYP3A4 inhibitors cause significant increases in atorvastatin concentrations (see Table 1 and related information below). Co-administration of potent CYP3A4 inhibitors (e.g., cyclosporine, telithromycin, clarithromycin, delavirdine, stiripentol, ketoconazole, voriconazole, itraconazole, posaconazole, and HIV protease inhibitors including ritonavir, lopinavir, atazanavir, indinavir, darunavir, etc.) should be avoided whenever possible. In cases where co-administration of these medicinal products with atorvastatin cannot be avoided, appropriate clinical monitoring of the patient is recommended (see Table 1).
Moderate CYP3A4 inhibitors (e.g., erythromycin, diltiazem, verapamil, and fluconazole) may increase plasma concentrations of atorvastatin (see Table 1). An increased risk of myopathy has been observed when erythromycin is coadministered with statins. Interaction studies evaluating the effect of amiodarone or verapamil on atorvastatin have not been conducted. Amiodarone and verapamil are known to inhibit CYP3A4 activity, and coadministration with atorvastatin may result in increased exposure to atorvastatin.
Therefore, appropriate clinical monitoring of the patient is recommended when co-administered with moderate CYP3A4 inhibitors. Appropriate clinical monitoring is recommended after initiation of treatment or after dose adjustment of the inhibitor.
CYP3A4 inducers.
Concomitant use of atorvastatin with cytochrome P450 3A inducers (such as efavirenz, rifampicin, St. John's wort) may result in variable decreases in plasma concentrations of atorvastatin. Due to the dual interaction of rifampicin (induction of cytochrome P450 3A and inhibition of the hepatic uptake transporter OATP1B1), simultaneous initiation of atorvastatin and rifampicin is recommended, as delayed administration of atorvastatin after administration of rifampicin is associated with a significant decrease in plasma concentrations of atorvastatin. However, the effect of rifampicin on atorvastatin concentrations in hepatocytes is unknown, therefore, in cases where concomitant use cannot be avoided, careful clinical monitoring of their efficacy in patients is recommended.
Inhibitors of transport proteins.
Inhibitors of transport proteins (e.g., cyclosporine) may increase systemic exposure to atorvastatin (see Table 1). The effect of inhibition of hepatic uptake transporters on atorvastatin concentrations in hepatocytes is unknown. In cases where concomitant use cannot be avoided, clinical monitoring of efficacy is recommended (see Table 1).
Gemfibrozil/fibroic acid derivatives.
The use of fibrates as monotherapy is sometimes associated with the occurrence of reactions from the muscular system, including rhabdomyolysis. The risk of such events increases with the simultaneous use of fibric acid derivatives and atorvastatin. In cases where concomitant use cannot be avoided, clinical monitoring of the patient is recommended (see section "Special instructions").
The use of ezetimibe as monotherapy is associated with the occurrence of muscular reactions, including rhabdomyolysis. The risk of such events is increased when ezetimibe and atorvastatin are used concomitantly. Appropriate clinical monitoring of these patients is recommended.
Colestipol.
When colestipol and atorvastatin were co-administered, plasma concentrations of atorvastatin and its active metabolites were decreased (by approximately 25%). However, the lipid effects were greater with the co-administration of atorvastatin and colestipol than with either drug administered as monotherapy.
Fusidic acid.
Interaction studies between atorvastatin and fusidic acid have not been conducted. As with other statins, muscle-related reactions, including rhabdomyolysis, have been reported in postmarketing experience with concomitant use of atorvastatin and fusidic acid. The mechanism of this interaction is unknown. Patients should be closely monitored and temporary interruption of atorvastatin therapy may be necessary.
Effect of atorvastatin on concomitantly used drugs.
Digoxin.
A small increase in steady-state digoxin concentrations was observed when multiple doses of digoxin were coadministered with 10 mg atorvastatin. Patients taking digoxin should be carefully monitored.
Oral contraceptives.
Concomitant use of atorvastatin with oral contraceptives leads to increased plasma concentrations of norethisterone and ethinyl estradiol.
Warfarin.
In a clinical study in patients receiving long-term warfarin therapy, coadministration of atorvastatin 80 mg daily with warfarin caused a small decrease in prothrombin time of approximately 1.7 seconds during the first 4 days of dosing, which returned to normal within 15 days of atorvastatin treatment. Although clinically significant anticoagulant interactions have been reported very rarely, prothrombin time should be determined prior to initiating atorvastatin therapy in patients receiving coumarin anticoagulants and frequently at the start of treatment to ensure that there are no significant changes in prothrombin time. Once stable prothrombin time has been achieved, monitoring can be performed at the frequency usually recommended for patients receiving coumarin anticoagulants. The same procedure should be repeated when Trinomia is discontinued. Atorvastatin therapy was not associated with bleeding or changes in prothrombin time in patients not taking anticoagulants.
Table 1. Effect of concomitant medication on the pharmacokinetics of atorvastatin
| Concomitant use of medications and dosage regimen | Atorvastatin | ||
| Dose (mg) | AUC changes (&) | Clinical recommendations (#) | |
| Tipranavir 500 mg, twice daily/ritonavir 200 mg, twice daily for 8 days (14th to 21st) | 40 mg on day 1, 10 mg on day 20 | ↑ 9.4 times | The use of the drug Trinomia is contraindicated in such cases. |
| Cyclosporine 5.2 mg/kg/day, stable dose | 10 mg once daily for 28 days | ↑ 8.7 times | |
| Lopinavir 400 mg, twice daily/ritonavir 100 mg, twice daily for 14 days | 20 mg once daily for 4 days | ↑ 5.9 times | There are no specific recommendations. Clinical monitoring of patients is recommended when the dose of atorvastatin exceeds 20 mg. |
| Clarithromycin 500 mg, 2 times a day for 9 days | 80 mg once daily for 8 days | ↑ 4.4 times | |
| Saquinavir 400 mg, twice daily/ritonavir (300 mg, twice daily from days 5 to 7, increased to 400 mg twice daily on day 8), and from days 5 to 18, 30 minutes after atorvastatin administration | 40 mg once daily for 4 days | ↑ 3.9 times | |
| Darunavir 300 mg, twice daily/ritonavir 100 mg, twice daily for 9 days | 10 mg once daily for 4 days | ↑ 3.3 times | |
| Itraconazole 200 mg, once daily for 4 days | 40 mg, single dose | ↑ 3.3 times | |
| Fosamprenavir 700 mg, twice daily/ritonavir 100 mg, twice daily for 14 days | 10 mg once daily for 14 days | ↑ 2.5 times | |
| Fosamprenavir 1400 mg, 2 times daily for 14 days | 10 mg once daily for 4 days | ↑ 2.3 times | |
| Nelfinavir 1250 mg, 2 times daily for 14 days | 10 mg once daily for 28 days | ↑ 1.7 times (^) | There are no special recommendations. |
| Grapefruit juice 240 ml, once a day* | 40 mg, single dose | ↑ 37% | The combined use of large amounts of grapefruit juice and atorvastatin is not recommended. |
| Diltiazem 240 mg, once daily for 28 days | 40 mg, single dose | ↑ 51% | Appropriate clinical monitoring of patients is recommended after initiation or dose adjustment of diltiazem. |
| Erythromycin 500 mg, 4 times a day for 7 days | 10 mg, single dose | ↑ 33% (^) | Appropriate clinical monitoring of patients is recommended. |
| Amlodipine 10 mg, single dose | 80 mg, single dose | ↑ 18% | |
| Cimetidine 300 mg, 4 times a day for 2 weeks | 10 mg once daily for 4 weeks | ↓ less than 1% | There are no special recommendations. |
| Antacid suspension of magnesium and aluminum hydroxide 30 ml, 4 times a day for 2 weeks | 10 mg once daily for 4 weeks | ↓ 35% (^) | There are no special recommendations. |
| Efavirenz 600 mg, once daily for 14 days | 10 mg for 3 days | ↓ 41% | There are no special recommendations. |
| Rifampin 600 mg once daily for 7 days (concomitant use) | 40 mg, single dose | ↑ 30% | In cases where concomitant use of atorvastatin with rifampin cannot be avoided, clinical monitoring of patients is recommended. |
| Rifampin 600 mg once daily for 5 days (divided doses) | 40 mg, single dose | ↓ 80% | |
| Gemfibrozil 600 mg twice daily for 7 days | 40 mg, single dose | ↑ 35% | Clinical monitoring of patients is recommended. |
| Fenofibrate 160 mg once daily for 7 days | 40 mg, single dose | ↑ 3% | Clinical monitoring of patients is recommended. |
(&) Data presented in times represent the simple ratio between co-administration and atorvastatin monotherapy (i.e. 1 time = no change). Data presented in percentages represent the differences relative to atorvastatin monotherapy (i.e. 0% = no change).
(#) See sections “Contraindications”, “Special instructions for use”, “Interaction with other medicinal products and other types of interactions” for determination of clinical significance.
(*) Contains one or more components that inhibit CYP3A4 and may increase plasma concentrations of drugs metabolized by CYP3A4. Consumption of 1 glass (240 ml) of grapefruit juice also caused a 20.4% decrease in the AUC of the active orthohydroxymetabolite. Large amounts of grapefruit juice (more than 1.2 L per day for 5 days) increased the AUC of atorvastatin by 2.5-fold and the AUC of its active metabolite.
(^) Full equivalent activity of atorvastatin.
Increase is indicated as "↑", decrease as "↓".
Table 2. Effect of atorvastatin on the pharmacokinetics of concomitantly administered drugs
| Atorvastatin dose and dosing regimen | Concomitantly used medications | ||
| Drug/dose (mg) | AUC changes (&) | Clinical recommendations | |
| 80 mg once daily for 10 days | Digoxin 0.25 mg once daily for 20 days | ↑ 15% | Appropriate monitoring should be performed in patients taking digoxin. |
| 40 mg once daily for 22 days | Oral contraceptive once daily for 2 months: - norethisterone 1 mg; - ethinylestradiol 35 mcg; | ↑ 28% ↑ 19% | There are no special recommendations. |
| 80 mg once daily for 15 days | * Phenazone, 600 mg, single dose | ↑ 3.0% | There are no special recommendations. |
(&) Data presented in percentages represent differences relative to atorvastatin monotherapy (i.e. 0% = no change).
(*) Coadministration of multiple doses of atorvastatin and phenazone showed little or no effect on the clearance of phenazone.
Increase is indicated as "↑", decrease as "↓".
Ramipril: pharmacodynamic and pharmacokinetic interactions.
Contraindicated combinations.
Extracorporeal treatments that result in blood coming into contact with negatively charged surfaces, such as dialysis or hemofiltration using certain high-affinity membranes
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.