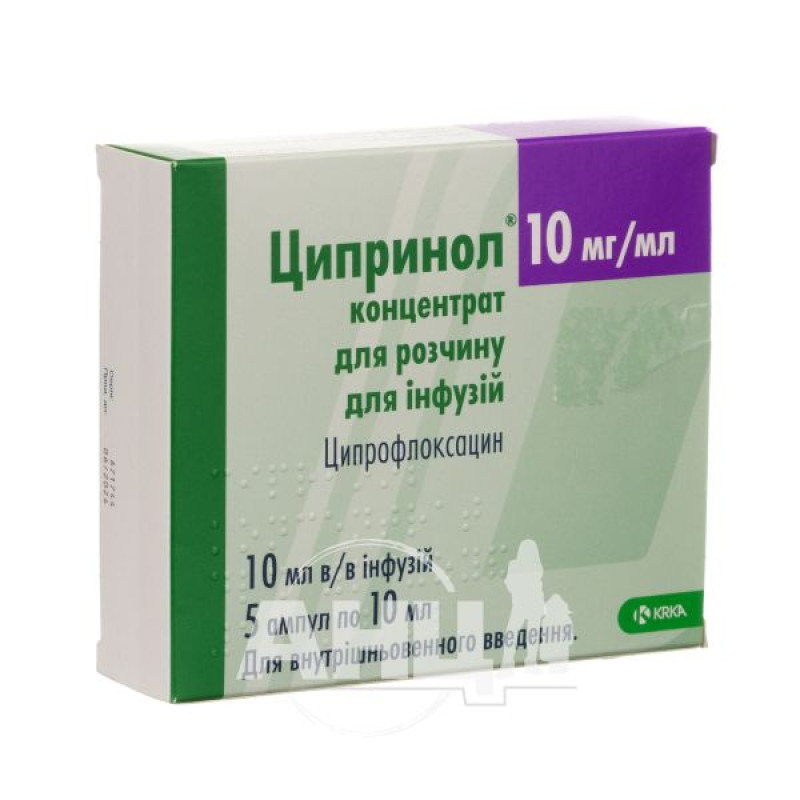
Tsiprinol concentrate for solution for infusions 100 mg ampoule 10 ml No. 5
Ciprofloxacin is indicated for the treatment of the following infections. Before initiating therapy, special attention should be paid to all available information on resistance to ciprofloxacin.
Official recommendations on the appropriate use of antibacterial drugs should be taken into account.
Adults
Lower respiratory tract infections caused by gram-negative bacteria: exacerbation of chronic obstructive pulmonary disease; bronchopulmonary infections in cystic fibrosis or bronchiectasis; pneumonia. Chronic purulent otitis media. Exacerbation of chronic sinusitis, especially if caused by gram-negative bacteria. Urinary tract infections. Infectious lesions of the reproductive system: epididymo-orchitis, in particular caused by Neisseria gonorrhoeae; pelvic inflammatory disease, in particular caused by Neisseria gonorrhoeae. Gastrointestinal tract infections (e.g. travelers' diarrhea). Intra-abdominal infections. Skin and soft tissue infections caused by gram-negative bacteria. Severe otitis externa. Bone and joint infections. Pulmonary anthrax (post-exposure prophylaxis and radical treatment).Ciprofloxacin can be used when administered to patients with neutropenia if there is a suspicion that the fever is caused by a bacterial infection.
Children and adolescents
Bronchopulmonary infections in cystic fibrosis caused by Pseudomonas aeruginosa Complicated urinary tract infections and pyelonephritis Pulmonary form of anthrax (post-exposure prophylaxis and radical treatment).Ciprofloxacin can be used to treat severe infections in children and adolescents when the doctor considers it necessary.
Treatment should only be initiated by a physician experienced in the treatment of cystic fibrosis and/or severe infections in children and adolescents (see sections “Special warnings and precautions for use” and “Pharmacological properties”).
Composition
active ingredient: 1 ml of concentrate for preparation of solution for infusions 10 mg of ciprofloxacin;
Excipients: lactic acid, sodium edetate, concentrated hydrochloric acid, water for injections.
Contraindication
Hypersensitivity to ciprofloxacin or to other components of the drug, as well as to other fluoroquinolones.
Concomitant use of ciprofloxacin and tizanidine (see section "Interaction with other medicinal products and other types of interactions").
Method of administration and doses
The dosage regimen is set individually depending on the localization and severity of the infection, the sensitivity of the pathogen, the patient's kidney function, and for children and adolescents - according to body weight.
The duration of treatment depends on the severity of the disease, clinical and bacteriological picture.
After starting treatment with intravenous administration, if the doctor considers it possible, the patient should switch to oral treatment (tablets).
Treatment of infections caused by certain bacteria (e.g. Pseudomonas aeruginosa, Acinetobacter or Staphylococci) may require administration of higher doses of ciprofloxacin and combination with other appropriate antibacterial agents.
Treatment of some infections (e.g. pelvic inflammatory disease, intra-abdominal infections, infections in neutropenic patients and bone and joint infections) may require combination with other appropriate antibacterial agents depending on the pathogen.
Ciprofloxacin should be administered by infusion. For children, the infusion duration is 60 minutes.
For adult patients, the infusion duration is 60 minutes for Ciprofloxacin 400 mg solution for infusion (200 ml) and 30 minutes for Ciprofloxacin 200 mg solution for infusion (100 ml). Slow infusion into a large vein will minimize patient discomfort and reduce the risk of venous irritation.
The concentrate should be diluted in a suitable infusion solution before use. The infusion solution should be administered either alone or after mixing with other compatible infusion solutions. The smallest volume is 50 ml.
Children
Ciprofloxacin is not recommended for use in children for the treatment of infectious diseases other than those listed in the "Indications" section.
Application features
Use during pregnancy or breastfeeding
Pregnancy.
Data on the use of ciprofloxacin in pregnant women demonstrate the absence of malformations or feto/neonatal toxicity. The possibility that the drug may be harmful to the articular cartilage of the newborn/fetus cannot be excluded. Therefore, as a precaution, it is better to avoid taking ciprofloxacin during pregnancy.
Breastfeeding period.
Ciprofloxacin passes into breast milk. Due to the potential risk of damage to articular cartilage in newborns, ciprofloxacin should not be used during breastfeeding.
Ability to influence reaction speed when driving vehicles or other mechanisms
Fluoroquinolones, to which ciprofloxacin belongs, may affect the patient's ability to drive or operate machinery due to nervous system reactions (see section "Adverse reactions"). Therefore, the ability to drive or operate other machinery may be impaired.
Overdose of 12 g has been reported to result in symptoms of moderate toxicity. Acute overdose of 16 g has resulted in acute renal failure.
Symptoms of overdose have included dizziness, tremor, headache, fatigue, convulsions, hallucinations, confusion, abdominal discomfort, renal and hepatic failure, as well as crystalluria and hematuria. Reversible renal toxicity has also been reported.
In addition to the usual emergency measures taken in case of overdose, it is recommended to monitor renal function, in particular, determine the pH of the urine and, if necessary, increase its acidity to prevent crystalluria. Patients should receive sufficient fluids. Antacids containing calcium or magnesium should theoretically reduce the absorption of ciprofloxacin in case of overdose.
Only a small amount of ciprofloxacin (<10%) is removed by hemodialysis or peritoneal dialysis.
In case of overdose, symptomatic treatment should be carried out. ECG monitoring is necessary, as the QT interval may increase.
Adverse reactions
The most commonly reported adverse reactions were nausea and diarrhea.
Interaction with other medicinal products and other types of interactions
Effect of other drugs on ciprofloxacin
Drugs that prolong the QT interval
Tsiprinol
®
As with other fluoroquinolones, it should be administered with caution to patients receiving drugs that prolong the QT interval (e.g. class IA and III antiarrhythmics, tricyclic antidepressants, macrolides, antipsychotics).
Storage conditions
Store in the original package in order to protect from light. This medicinal product does not require any special temperature storage conditions. Keep out of the reach of children.
Shelf life - 5 years.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.