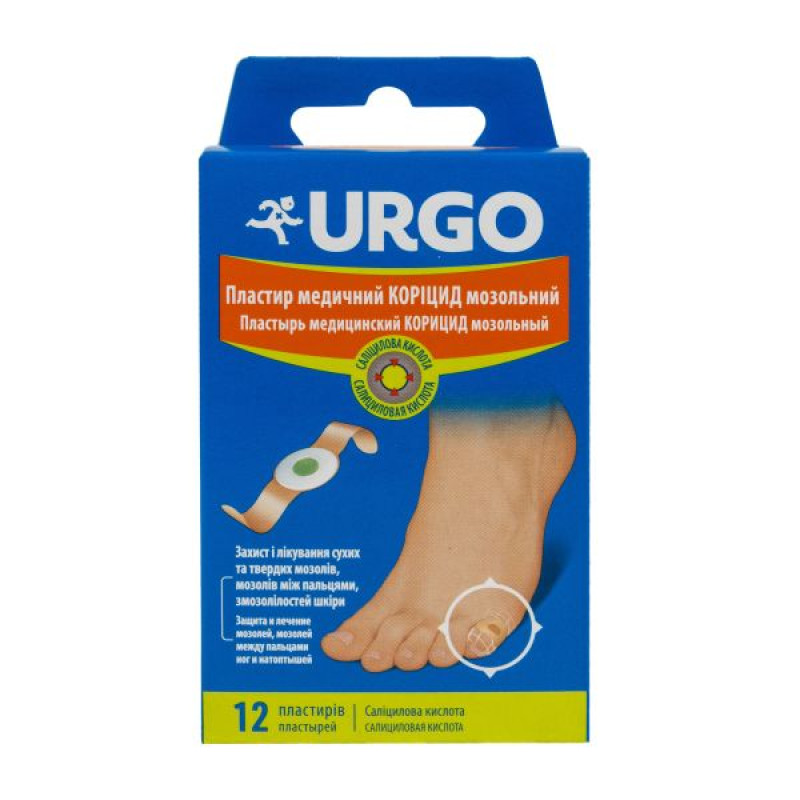
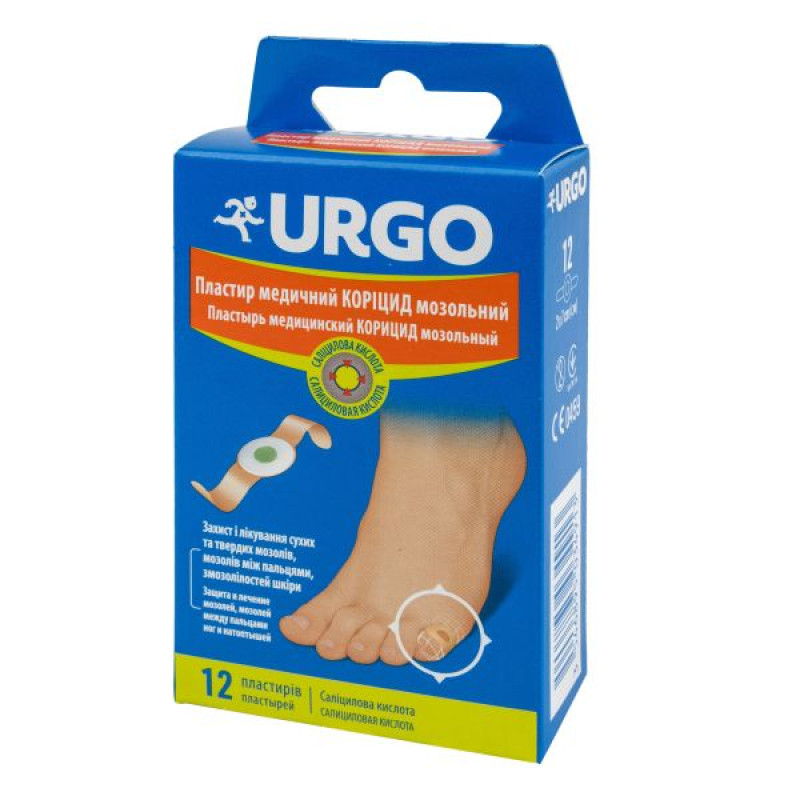
Urgo medical plaster coricide callus No. 12
Instructions for Urgo medical plaster coricide callus No. 12
Description
International name: salicylic acid;
Main physicochemical properties: the patch consists of a flesh-colored adhesive base with a white foamed polyethylene disc fixed in the center, which contains a green mass; the adhesive part of the patch and the central disc are covered with protective paper;
Composition
1 patch contains 32 mg of salicylic acid;
excipients: white beeswax, macrogol lauroylglyceride (Labrafil M2130CS®), colloidal hydrophobic silicon dioxide (Aerosil R972®), copper chlorophyll.
Release form
Medical adhesive plaster.
Pharmacotherapeutic group
A remedy for removing calluses and warts. ATS code D11AF.
Pharmacological properties
Pharmacodynamics
URGOKOR CALCULATOR acts as a keratolytic, dissolving the keratinized layer of skin in the area of calluses.
Pharmacokinetics
Not studied.
Indications for use
URGOKOR CALCULUS is indicated for the topical treatment of dry and hard calluses and skin calluses in adults.
Method of administration and doses
Skin applications. After a hot foot bath, dry the affected area thoroughly, attach a patch so that the disc with salicylic paste covers the callus.
Avoid covering the healthy skin around the callus. Change the patch daily.
The duration of treatment depends on the results obtained.
Side effect.
When applied to healthy skin, local irritation in the form of itching is possible. In some cases, allergic reactions may develop.
Contraindication
- Hypersensitivity to salicylic acid, similar substances or other components of the drug;
- infected corns.
Overdose
Given the characteristics of the dosage form, overdose is impossible.
Application features
Salicylic acid, which is part of the preparation, is a caustic substance. Do not use on blisters. It is not recommended to apply the patch to healthy skin around the affected area. If pain occurs, the use of the patch should be discontinued.
Patients with arthritis, diabetes, and neuropathy should consult a doctor before using the URGOKOR CALCULATOR patch.
If treatment is unsuccessful, the treatment approach should be changed. It may be useful to identify the causes of the disorders (use orthopedic measures, change shoes).
There is no experience with the use of the URGOKOR CALCULUS patch in children.
Pregnancy and lactation
There is no data on the negative effects of the patch during pregnancy or breastfeeding.
Effects on ability to drive and use machines
Does not affect.
Interaction with other drugs
Given the specifics of the dosage form and purpose of the drug, there are no data on interactions.
Conditions and shelf life
Store at room temperature (15-25°C) out of the reach of children.
Expiration date
3 years.
Vacation conditions
Without a prescription.
Packaging
6 or 12 patches in paper bags, connected in pairs and separated by perforation, in a cardboard box.
Producer
Laboratoires URGO
Address
42, rue de Longvic- 21300 Chenove – France / 42, rue de Longvic- 21300 Chenove – France
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.