Uromune-MB140 sublingual spray 300 FTU/ml bottles with sprayer 9 ml No. 2
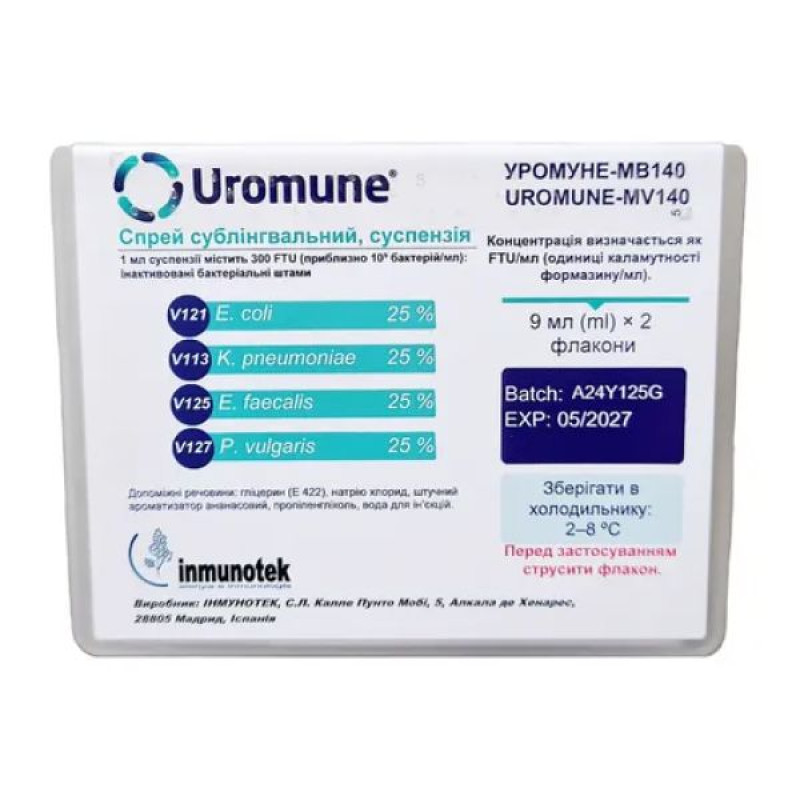
Instructions for use Uromune-MB140 sublingual spray 300 FTU/ml bottles with sprayer 9 ml No. 2
Composition
active ingredients: 1 ml of suspension contains 300 FTU (approximately 109 bacteria/ml): inactivated bacterial strains 25% Escherichia coli (V121), 25% Klebsiella pneumoniae (V113), 25% Enterococcus faecalis (V125) and 25% Proteus vulgaris (V127);
excipients: glycerin (E 422), sodium chloride, artificial pineapple flavor, propylene glycol, water for injections.
The concentration is expressed as FTU/ml (formazine turbidity units/ml).
Dosage form
Sublingual spray, suspension.
Pharmacotherapeutic group
Anti-infectives for systemic use. Bacterial vaccines. Other bacterial vaccines. ATX code J07AX.
Immunological and biological properties
Pharmacodynamics.
Mechanism of action
UROMUNE-MB140 is a vaccine that stimulates the immune system to increase resistance to urinary tract infections.
The drug UROMUNE-MB140 is intended for secondary prevention of recurrent bacterial urinary tract infections in adult patients.
The active substance of the drug UROMUNE-MB140 is bacteria that are the main cause of urinary tract infections (UTIs). The bacteria contained in the drug are completely inactivated and cannot enter the bloodstream from the oral mucosa. Most likely, they are actively taken up by oral dendritic cells. These cells induce an adaptive local immune response (site of induction), which spreads to other mucosal tissues, in particular in the bladder (site of effect).
Sublingual vaccination with UROMUNE-MB140 stimulates a specific immune response (Th17/Th1), a major mechanism involved in resistance to bacterial bladder infections. It also induces a regulatory T-cell response that, together with the Th1 response, suppresses the deleterious Th2 responses associated with recurrent bladder infections. These responses were observed in vitro and in vivo in preclinical studies. In vivo studies have demonstrated these responses in the spleen and distant (inguinal) lymph nodes following sublingual administration. Early TNF-α responses are also observed in the bladder of mice immunized sublingually after challenge with bacteria contained in UROMUNE-MB140. In an experimental model of Escherichia coli bladder infection, UROMUNE-MB140 provided early protection that was associated with a rapid T-cell response in the bladder along with an increase in the influx of myeloid cells into the urine. In addition, UROMUNE-MB140 elicited antibody responses, including immunoglobulins A, G, and M, against the bacteria it contained.
Its pharmacodynamic effect is directed at the immune system.
Clinical efficacy and safety
The efficacy and safety of UROMUNE-MB140 in recurrent bacterial urinary tract infections was evaluated in a multicenter, randomized, double-blind, placebo-controlled, one-year, parallel-group, phase III study involving 240 women (aged 18 to 75 years). Women who had had at least 5 episodes of uncomplicated cystitis in the previous year were enrolled and received either placebo or UROMUNE-MB140 for 3 or 6 months. The follow-up period was 12 months from the start of treatment.
During the 9-month efficacy evaluation period (i.e., after 3 months of use), the median number of UTI episodes was 3.0 [interquartile range, IQR, 0.5–6.0] in the placebo group compared to 0.0 [IQR, 0.0–1.0] in both groups treated with UROMUNE-MB140 (p < 0.001). In addition, there was a significant increase in the incidence of UTI-free events (p < 0.001), which was 25% in the placebo group compared to 55.7% and 58% in the patients treated with UROMUNE-MB140 for 3 or 6 months, respectively. The median time to first UTI was significantly prolonged in those treated with UROMUNE-MB140, at least 227 days. A highly significant reduction in symptom scores was also observed, along with a significant improvement in quality of life, throughout the study period when the active treatment group was compared with the placebo group (p < 0.001). The median antibiotic score was 4.5 [IQR, 1.0–8.5] for the placebo group and decreased to 1.0 [IQR, 0.0–3.0] in both groups receiving UROMUNE-MB140 (p < 0.001) throughout the study period. In addition, there was a significant reduction in healthcare resource utilization, mainly related to urologist visits and additional tests. There were no differences between the active treatment schedules on any clinical variable.
Regarding safety, only 5 patients (2.2%) reported non-serious adverse reactions: 2 in the placebo group and 3 in the UROMUNE-MB140 group over 3 months.
In various observational studies, prophylaxis with UROMUNE-MB140 has been shown to be more effective in treating recurrent bacterial urinary tract infections than prophylaxis with antibacterial agents, with a reduction in the incidence of UTIs and an increase in the infection-free period. Over a 15-month follow-up period (p < 0.001 for all studies), subjects who received UROMUNE-MB140 daily for 3 months (total 519 women) had significantly higher UTI-free rates (35–90%) than those who received 6 months of antibiotic prophylaxis (0% of 499 women).
Women with recurrent bacterial urinary tract infections who had failed conventional therapy were treated with UROMUNE-MB140 for 3 months. The majority of participants (78%) were free of infection after a 12-month follow-up period, suggesting that UROMUNE-MB140 is effective in preventing recurrent UTIs in women. Another prospective study involving nearly 800 subjects showed that UROMUNE-MB140 significantly reduced the number of UTI episodes in both men and women. And a recent study in women with various risk factors for UTIs showed that UROMUNE-MB140 provided protection that was maintained, at least in part, for 2 years after treatment. In this study, the drug UROMUNE-MB140 significantly reduced antibiotic use and healthcare costs associated with UTIs.
Other studies evaluating subjects with complicated UTIs have consistently reported favorable UTI-free rates ranging from 30 to 50%, significant reductions in UTI incidence, and improvements in quality of life. One of these studies included patients with autoimmune diseases, where immunosuppressive therapy predisposes them to episodes of infection. Both the number of UTI episodes, antibiotic use, and healthcare resource requirements, assessed 12 months after the start of a 3-month course of treatment with UROMUNE-MB140, were significantly reduced compared with the year before vaccination.
Some preliminary studies involving women aged 16–18 years and various case reports in children aged 6–12 years, based on real-life clinical experience, have demonstrated clinical benefits of UROMUNE-MB140 in children. Most children reported no recurrence or reduced frequency of UTIs, and reduced severity of infection episodes (asymptomatic bacteriuria or no febrile UTIs).
The above studies were systematically reviewed and concluded that UROMUNE-MB140 reduces UTIs by 2–6-fold over 12 months, with almost 90% of patients free of infection.
A study of elderly patients shows that after bacterial immunoprophylaxis, the number of UTIs 12 months after treatment with autovaccine or UROMUNE-MB140 was significantly reduced in both men and women in all treatment groups compared with the number of cases before vaccination. The average number of UTIs after prophylaxis ranged from 0.0 to 0.3 episodes per month depending on the study group.
Overall, the rate of reduction ranged from 7-fold in subjects receiving a new course of autovaccine to 40-fold in women who were first treated with UROMUNE-MB140. It is worth noting that in all groups, a significantly greater reduction in UTIs was observed in subjects receiving UROMUNE-MB140 compared with those receiving autovaccines. Regarding the proportion of subjects who were free of urinary tract infections, almost 60% of subjects receiving UROMUNE-MB140 were free of urinary tract infections at the end of the follow-up period, but none of the autovaccination groups. At the end of the study, there were no differences in the number of UTIs between the patient groups by gender, as well as between the primary treatment and autovaccination groups.
Several studies of UROMUNE-MB140 as a prophylactic treatment for patients with recurrent bacterial urinary tract infections have reported good tolerability and no adverse reactions during and after 3 months of daily administration of UROMUNE-MB140. In post-marketing studies involving thousands of cases of UROMUNE-MB140 therapy, 13 spontaneous reports of adverse reactions were reported worldwide over the past 6 years of use of UROMUNE-MB140.
Pharmacokinetics.
Due to the method of administration, the active substances (inactivated bacterial strains) are not absorbed into the vascular system. Therefore, animal pharmacokinetic studies or clinical studies of the pharmacokinetic profile and metabolism of the drug UROMUNE-MB140 have not been conducted.
Preclinical safety
Non-clinical data reveal no special hazard for humans based on conventional studies of repeated dose toxicity, local tolerability, and safety of use assessed during pharmacodynamic/mechanism of action studies.
Indication
An immunotherapeutic drug indicated in adults for the prevention of recurrent bacterial urinary tract infections.
Contraindication
Hypersensitivity to the components of the drug (see the "Composition" section).
Children's age (up to 18 years).
Pregnancy or breastfeeding.
Interaction with other medicinal products and other types of interactions
Interactions with other drugs have not been studied.
Application features
The medicine is prescribed exclusively by a doctor.
Any unused product or waste material should be disposed of in accordance with local requirements.
The medicine contains completely inactivated microorganisms, therefore it has no infectious potential.
This medicine contains less than 1 mmol sodium (23 mg) per ml, i.e. essentially ‘sodium-free’.
Use during pregnancy or breastfeeding
Pregnancy
Data on the use of the drug UROMUNE-MB140 in pregnant women are absent or limited, therefore this drug is not recommended for use during pregnancy.
Breast-feeding
There is insufficient information on the effects of UROMUNE-MB140 on newborns/infants, therefore it is not recommended to prescribe this medicine during breastfeeding.
Fertility
Data is missing.
Ability to influence reaction speed when driving vehicles or other mechanisms
Allergen preparations do not affect the ability to drive or use other mechanisms.
Method of administration and doses
The recommended dose is 2 sprays per day per procedure (one dose – 2 sprays), the maximum daily dose is 0.2 ml. The duration of treatment is approximately 90 days (3 months).
UROMUNE-MB140 should be taken separately from food and/or drinks (at least 30 minutes before or after eating food and/or drinks). Do not brush your teeth or rinse your mouth for 30 minutes before or after taking it.
Method of application
UROMUNE-MV140 is administered sublingually.
- Remove the plastic cap from one bottle.
- Shake the bottle gently.
- Turn the nozzle to the side. Press 3 or 4 times to prime the pump mechanism (only when activating the bottle).
- Lift your tongue, point the nozzle under your tongue and spray twice to apply the appropriate dose.
- Hold the medicine under your tongue for about 1–2 minutes before swallowing.
- Return the nozzle back to its original vertical position, thereby locking the spray mechanism.
- Return the vial to the carton and store as described in the “Storage Conditions” section.
Children.
Do not use in children (under 18 years of age).
Overdose
There are still no known cases of overdose with the drug UROMUNE-MV140. Due to the characteristics of the drug, overdose is unlikely.
In case of accidental overdose or incorrect use, patients may experience some adverse reactions (see section "Adverse reactions").
Adverse reactions
Rarely, gastrointestinal disturbances, oropharyngeal discomfort and/or sensation may occur. The presence of these reactions does not mean that treatment should be interrupted or postponed, but monitoring of the drug intake may be necessary.
Information on the adverse reactions listed below was obtained from clinical trials, spontaneous reports and the medical literature.
Adverse reactions are classified by frequency: very common (≥ 1/10); common (≥ 1/100 to < 1/10); uncommon (≥ 1/1000 to < 1/100); rare (≥ 1/10000 to < 1/1000); very rare (< 1/10000), unknown (cannot be estimated from the available data).
All side effects should be reported to the doctor.
| Organ classes and systems | Very often | Often | Infrequently | Rarely | Very rare |
| Metabolism and nutrition | Decreased appetite | ||||
| From the nervous system | Formication (tactile hallucinations in the form of a crawling sensation, ants or other insect bites) Headache | ||||
| From the organs of vision | Eye pain | ||||
| Respiratory system | Asthma/asthma exacerbation Upper respiratory tract cough syndrome Dyspnea | ||||
| Gastrointestinal tract | Pain in the oral cavity Feeling of discomfort in the epigastric area Exfoliation of the oral mucosa Discomfort in the teeth Dry mouth Gastritis | Abdominal discomfort Abdominal pain Glossitis Nausea Diarrhea Gastroesophageal reflux | |||
| Skin and subcutaneous tissue disorders | Itch | Rash Itchy rash Perioral dermatitis | |||
| Musculoskeletal system | Arthralgia Pain in the extremities | ||||
| General disorders and administration site conditions | Malaise | Feeling hot Generalized edema Pain Hot flashes |
Most adverse reactions are rare or very rare. They may be local (at the injection site) or systemic.
Rarely, systemic adverse reactions such as malaise, rash, generalized itching may occur. In addition, a very small number of cases of asthma or exacerbation of asthma have been reported, and in the event of the latter, treatment should be discontinued and it is strongly recommended to inform the doctor.
Reporting of suspected adverse reactions
Reporting adverse reactions after the registration of a medicinal product is important. This allows monitoring of the benefit/risk ratio of the medicinal product. Medical and pharmaceutical professionals, as well as patients or their legal representatives, should report all cases of suspected adverse reactions and lack of efficacy of the medicinal product via the Automated Information System for Pharmacovigilance at the link: https://aisf.dec.gov.ua/.
Expiration date
3 years.
Shelf life after first opening the bottle is 45 days.
Storage conditions
Store in original packaging in a dark place.
Store in a refrigerator (2–8°C). Keep out of the reach of children.
Packaging
2 bottles of 9 ml, closed with a plastic applicator with a built-in sprayer, in a plastic box.
Vacation category
According to the recipe.
Producer
IMMUNOTEK, S.L
Location of the manufacturer and address of its place of business.
Calle Punto Mobi, 5, Alcala de Henares, 28805 Madrid, Spain
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.