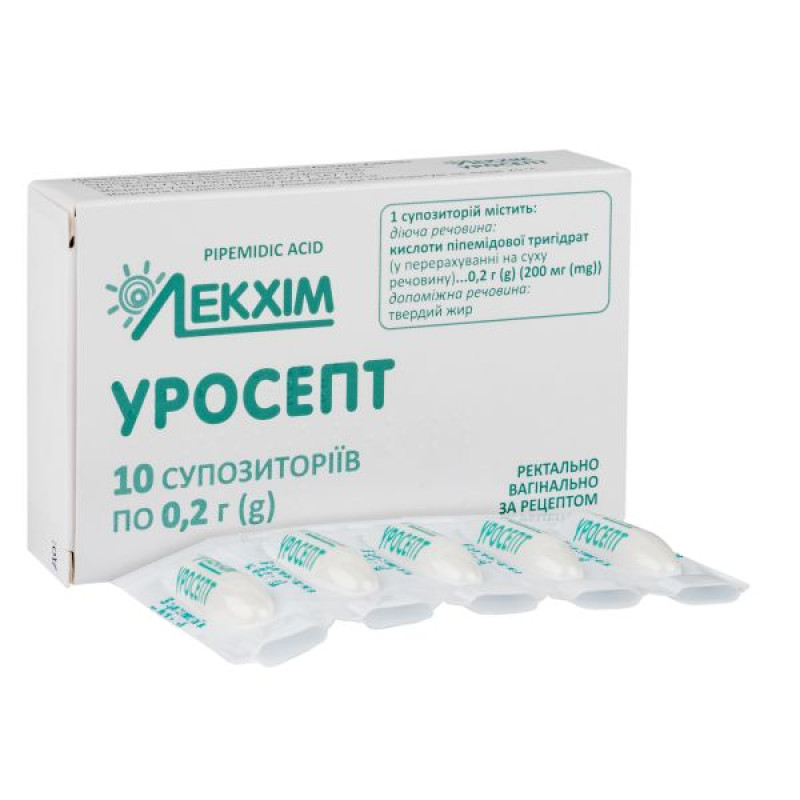
Urosept suppositories 0.2 g blister No. 10

Instructions for use Urosept suppositories 0.2 g blister No. 10
Composition
active ingredient: ripemidic acid;
1 suppository contains pipemidic acid trihydrate in terms of dry matter 0.2 g (200 mg);
excipient: solid fat.
Dosage form
Suppositories.
Main physicochemical properties: suppositories are white or white with a yellowish tinge, spherical in shape. The presence of a coating on the surface of the suppository is allowed.
Pharmacotherapeutic group
Antibacterial agents of the quinolone group.
ATX code J01M B04.
Pharmacological properties
Pharmacodynamics
A quinolone urinary antiseptic. It has a bactericidal effect, active against most gram-negative microorganisms, as well as against some gram-positive microorganisms, in particular Staphylococcus aureus.
Pharmacokinetics
The maximum concentration of the drug in the blood plasma is determined 2-3 hours after administration and coincides in time with the maximum concentration of the drug in the urine. It is excreted by the kidneys unchanged.
Indication
Infectious-inflammatory diseases caused by microorganisms sensitive to pipemidic acid, including pyelonephritis, urethritis, cystitis, prostatitis, nonspecific colpitis.
Contraindication
Hypersensitivity to pipemidic acid, quinolones or any other component of the drug. Severe renal impairment (creatinine clearance less than 10 ml/min), severe hepatic impairment. Cirrhosis of the liver, porphyria. Central nervous system diseases (epilepsy, neurological conditions with a reduced seizure threshold).
Interaction with other medicinal products and other types of interactions
With prolonged use of pipemidic acid, the half-life of theophylline is prolonged, so its serum concentration increases by 40-80%. Therefore, patients taking theophylline should have their serum levels monitored more frequently.
Quinolones increase serum caffeine concentrations; pipemidic acid can provide a 2 to 4-fold increase.
The drug may enhance the effect of warfarin, rifampicin, and cimetidine when used simultaneously.
The simultaneous use of quinolones and nonsteroidal anti-inflammatory drugs increases the risk of seizures.
Antacids (aluminum, magnesium, and calcium preparations) and sucralfate significantly reduce the absorption of pipemidic acid and should not be administered simultaneously. The interval between administrations of these drugs should be 2-3 hours. However, this effect was not observed with simultaneous use with cimetidine and ranitidine.
A synergistic bactericidal effect occurs with aminoglycosides.
Application features
During treatment, patients should drink plenty of fluids.
In rare cases, Urosept may cause seizures, therefore, treatment is contraindicated for patients with epilepsy and other neurological diseases with a reduced seizure threshold.
Due to the risk of acute porphyrin crisis, treatment of patients with porphyria is contraindicated.
The drug should be prescribed with caution to patients over 70 years of age, as side effects are more common in the elderly.
The patient should be warned to avoid direct sunlight and artificial ultraviolet radiation during treatment with pipemidic acid due to possible photosensitization.
Superinfections caused by resistant bacteria and fungi may develop.
The drug should be used with caution in the treatment of patients with glucose-6-phosphate dehydrogenase deficiency, as quinolones can cause acute hemolytic crisis.
With prolonged treatment with pipemidic acid, pseudomembranous colitis may develop, therefore, if the patient develops diarrhea, appropriate measures should be taken.
Treatment with pipemidic acid may alter the intestinal flora, resulting in an increase in the number of Clostridium difficile bacteria, and, consequently, pseudomembranous colitis may develop.
In adolescents, taking the drug may affect temporary cartilage tissue, so the use of pipemidic acid is contraindicated in children under 18 years of age.
Allergic reactions most often occur in people with hypersensitivity to acetylsalicylic acid.
Laboratory tests: A false-positive reaction to glucose in the urine may occur.
Use during pregnancy or breastfeeding
The drug is contraindicated for use during pregnancy or breastfeeding.
Ability to influence reaction speed when driving vehicles or other mechanisms
When using the drug, you should refrain from driving or operating other mechanisms, as dizziness and visual disturbances are possible (see the "Adverse Reactions" section).
Method of administration and doses
Before using the suppository, you must:
tear off 1 suppository from the primary packaging along the perforation line of the blister pack;
Apply 1 suppository vaginally or rectally 2 times a day for 10 days. If necessary, increase the daily dose to 3 suppositories, i.e. 1 suppository 3 times a day.
Children
The drug should not be used to treat children.
Overdose
Symptoms: nausea, vomiting, dizziness, headache, confusion, tremors and convulsions.
Treatment. If the patient has not lost consciousness as a result of taking a large amount of the drug, it is recommended to induce vomiting, rinse the stomach, and administer activated charcoal.
Pipemidic acid is removed by hemodialysis (90% in 6 hours).
If side effects from the central nervous system occur (including epileptiform convulsions), prescribe symptomatic treatment (diazepam).
Side effects
Local reactions: irritation, pain, itching at the site of drug administration.
Systemic reactions:
Blood and lymphatic system disorders: eosinophilia is rare, and reversible thrombocytopenia has been observed in elderly patients and patients with renal impairment. Hemolytic anemia is possible in individuals with glucose-6-phosphate dehydrogenase deficiency;
mental disorders: agitation, depression, confusion, hallucinations;
Nervous system: tremor, sleep disturbances, sensory disturbances, dizziness; very rarely - convulsions, vertigo, headache;
On the part of the organs of vision: visual impairment;
Skin and subcutaneous tissue disorders: hypersensitivity reactions including skin rash, pruritus, photosensitivity, Stevens-Johnson syndrome; rarely, angioedema. Skin reactions are reversible. There have been reports of anaphylactic reactions. Due to the possibility of cross-sensitivity to other quinolones, caution is required in treating patients who have had an anaphylactic reaction to any quinolone;
Musculoskeletal system: acute arthropathy, tendinitis;
Gastrointestinal: anorexia, epigastric pain, heartburn, nausea, vomiting, flatulence, abdominal pain, diarrhea or constipation; rarely - pseudomembranous colitis;
others: rarely - weakness, development of resistance, superinfection.
If signs of hypersensitivity reactions, anaphylactic shock, toxic epidermal necrolysis or convulsions occur, treatment should be discontinued immediately.
Expiration date
3 years.
Storage conditions
Keep out of reach of children. Store in original packaging at a temperature not exceeding 25 °C.
Packaging
5 suppositories in a blister; 2 blisters in a pack.
Vacation category
According to the recipe.
Producer
Private Joint-Stock Company "Lekhim-Kharkiv".
Location of the manufacturer and address of its place of business
Ukraine, 61115, Kharkiv region, Kharkiv city, Severyna Pototskoho street, building 36.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.