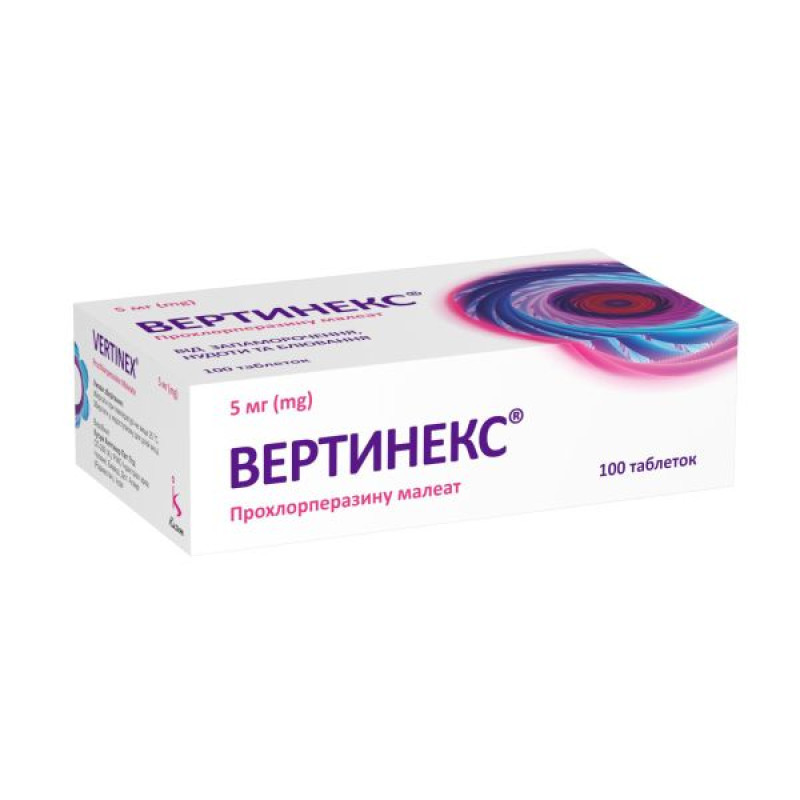
Vertinex tablets 5 mg blister No. 100
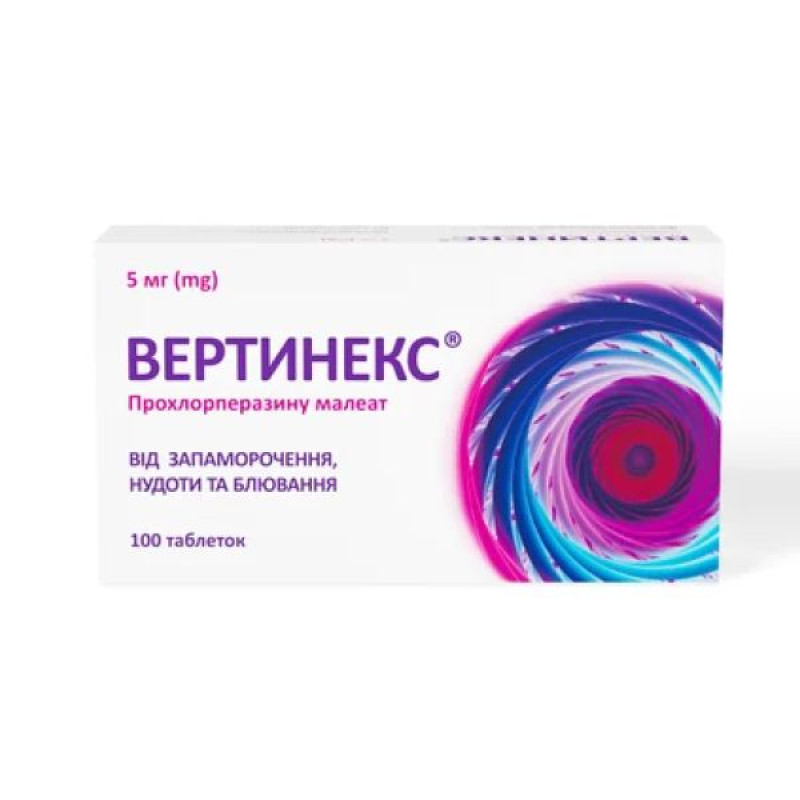
Instructions Vertinex tablets 5 mg blister No. 100
Composition
active ingredient: prochlorperazine maleate;
1 tablet contains prochlorperazine maleate 5 mg;
Excipients: lactose monohydrate, microcrystalline cellulose, corn starch, croscarmellose sodium, sodium lauryl sulfate, magnesium stearate, colloidal anhydrous silicon dioxide.
Dosage form
Pills.
Main physicochemical properties: white or almost white, round, biconvex tablets, smooth on both sides.
Pharmacotherapeutic group
Antipsychotics. Phenothiazines with a piperazine structure. ATX code N05A B04.
Pharmacological properties
Pharmacodynamics
Prochlorperazine maleate is a phenothiazine derivative.
Prochlorperazine has a broad spectrum of activity due to its CNS depressant, alpha-adrenergic blocking, and weak antimuscarinic effects. It inhibits dopamine and prolactin-inhibitory factor, thereby stimulating prolactin release and accelerating dopamine metabolism in the brain. There is evidence that the therapeutic effect in psychiatric conditions is due to the antagonistic effect of prochlorperazine on dopaminergic receptors in the CNS.
Prochlorperazine has a sedative effect, but in most cases tolerance develops quickly. Prochlorperazine has antiemetic and antipruritic effects and blocks the action of serotonin. In addition, prochlorperazine has a slight antihistamine effect and a weak blocking effect on the ganglia. Prochlorperazine also has an inhibitory effect on the thermoregulatory center, a relaxing effect on smooth muscles and has membrane-stabilizing and local anesthetic properties. The effect of prochlorperazine on the autonomic nervous system leads to vasodilation, arterial hypotension, tachycardia, hyposalivation and decreased secretion of gastric juice.
Pharmacokinetics
Prochlorperazine is well absorbed from the gastrointestinal tract, but undergoes extensive presystemic metabolism in the intestinal wall. It is also extensively metabolized in the liver and excreted in the urine and bile. After oral administration of prochlorperazine, its plasma concentration is significantly lower than after intramuscular administration. There is no direct correlation between the plasma concentration of prochlorperazine and its metabolites and the therapeutic effect.
Prochlorperazine can be metabolized by hydroxylation and conjugation with glucuronic acid, N-oxidation, sulfur atom oxidation and dealkylation. The plasma half-life is only a few hours, but the elimination of metabolites can be very long. Prochlorperazine is highly bound to plasma proteins and is well distributed throughout the body, its metabolites cross the placental barrier and enter breast milk. The rate of metabolism and excretion of prochlorperazine is reduced in elderly patients.
Indication
Dizziness caused by Meniere's syndrome, inflammation of the inner ear, and other causes.
Nausea and vomiting, occurring for any reason, including migraine.
It can also be used as an additional medication for the short-term treatment of anxiety conditions.
Contraindication
Known hypersensitivity to prochlorperazine or to other components of the drug.
Interaction with other medicinal products and other types of interactions
In case of overdose with prochlorperazine, adrenaline should not be used (see section "Overdose").
The CNS depressant effect of prochlorperazine may be potentiated (additively) by alcohol, barbiturates and other sedatives. Respiratory depression may occur.
Anticholinergic drugs may reduce the antipsychotic effect of prochlorperazine.
The moderate anticholinergic effect of prochlorperazine may be potentiated by other anticholinergic drugs, which may lead to constipation, heat stroke, etc.
Antacids, antiparkinsonian drugs and lithium preparations may impair the absorption of prochlorperazine.
If treatment of extrapyramidal symptoms caused by neuroleptics is necessary, anticholinergic antiparkinsonian agents should be preferred to levodopa, since neuroleptics counteract the antiparkinsonian effect of dopaminergic agents.
High doses of neuroleptics reduce the hypoglycemic effect of blood sugar-lowering drugs, so their dose may need to be adjusted.
Neuroleptic drugs may enhance the hypotensive effect of most antihypertensive drugs, especially alpha-blockers.
Prochlorperazine, like other phenothiazine neuroleptics, may inhibit the effects of certain drugs: amphetamine, levodopa, clonidine, guanethidine, and adrenaline.
There is evidence of changes in plasma concentrations of some drugs (e.g. propranolol, phenobarbital), which is not clinically significant.
There is an increased risk of arrhythmia with the concomitant use of prochlorperazine and drugs that prolong the QT interval (including some antiarrhythmic drugs, antidepressants and other antipsychotics), and drugs that disrupt electrolyte balance.
There is an increased risk of agranulocytosis with the concomitant use of prochlorperazine and drugs with myelosuppressive potential (such as carbamazepine, certain antibiotics and cytotoxic agents).
Neurotoxic effects have been reported rarely with the concomitant use of neuroleptics and lithium preparations.
Application features
Prochlorperazine should not be used in patients with impaired hepatic or renal function, Parkinson's disease, hypothyroidism, heart failure, pheochromocytoma, myasthenia gravis, and prostatic hypertrophy. Prochlorperazine should not be administered to patients with known hypersensitivity to prochlorperazine, patients with angle-closure glaucoma, or a history of agranulocytosis.
Patients with epilepsy or a history of seizures should be carefully monitored because prochlorperazine may lower the seizure threshold.
Since cases of agranulocytosis have been reported with prochlorperazine, regular monitoring of complete blood counts is recommended. The development of an unexplained infection or fever in a patient may indicate a blood dyscrasia, and appropriate hematological investigations should be performed.
Treatment with prochlorperazine should be discontinued if fever of unknown origin occurs, as it may be a sign of the development of neuroleptic malignant syndrome (pallor, hyperthermia, autonomic dysfunction, impaired consciousness, muscle rigidity). Symptoms of autonomic dysfunction, such as hyperhidrosis and unstable blood pressure, may precede the appearance of hyperthermia and serve as early signs of neuroleptic malignant syndrome. Although this syndrome may also be idiosyncratic in origin, factors contributing to its development include dehydration and organic brain disease.
Since acute withdrawal symptoms (including nausea, vomiting, insomnia, extrapyramidal reactions) have been reported after abrupt discontinuation of high doses of neuroleptics, gradual withdrawal of prochlorperazine is advisable.
Prochlorperazine, like other neuroleptic phenothiazines, may potentiate QT prolongation, which increases the risk of torsades de pointes, which is potentially fatal (sudden death). The risk of QT prolongation is increased in patients with bradycardia, hypokalemia, or congenital or drug-induced QT prolongation. Therefore, a full risk-benefit assessment should be made before prescribing prochlorperazine. It is recommended that appropriate clinical and laboratory examinations (e.g. blood chemistry and ECG) be performed before and during prochlorperazine therapy and during the initial phase of treatment as necessary during treatment to exclude possible risk factors (such as cardiac disease, family history of QT prolongation, metabolic disorders such as hypokalemia, hypocalcemia or hypomagnesemia, history of fasting, alcohol abuse, concomitant therapy with other drugs that prolong the QT interval) (see also sections “Interaction with other medicinal products and other forms of interaction” and “Adverse reactions”).
Concomitant therapy with other neuroleptics should be avoided (see section “Interaction with other medicinal products and other types of interactions”).
Stroke: In randomized clinical trials conducted in elderly patients with dementia treated with some atypical antipsychotics, a three-fold increased risk of cerebrovascular events was observed compared with patients treated with placebo. Since the mechanism of this risk is unknown, it cannot be excluded with other antipsychotics or in other patient populations. Prochlorperazine should be used with caution in patients with risk factors for stroke.
Like other antipsychotics, prochlorperazine should not be used as monotherapy in patients with predominantly depressive symptoms. However, it can be added to antidepressant therapy to treat conditions where depression and psychosis coexist.
Due to the risk of photosensitivity, patients taking prochlorperazine should avoid direct sunlight.
To prevent skin sensitization in individuals who frequently come into contact with phenothiazine derivatives, they should avoid skin contact with the drug (see section "Adverse reactions").
Old age.
Increased mortality rates in elderly patients with dementia.
There is evidence of a small increased risk of death in elderly patients with dementia treated with antipsychotics. As there are insufficient data to assess the magnitude and cause of this risk, prochlorperazine should not be prescribed for the treatment of behavioral disturbances associated with dementia.
Increased risk of developing venous thromboembolism (VTE).
Cases of VTE have been reported with antipsychotic drugs. Since patients receiving antipsychotic drugs often have risk factors for VTE, these should be identified and appropriate preventive measures taken before and during treatment with prochlorperazine.
Hyperglycemia or impaired glucose tolerance.
Cases of hyperglycemia or impaired glucose tolerance have been reported in patients receiving phenothiazine antipsychotics. Patients with established diabetes mellitus or those with risk factors for diabetes require appropriate glycemic control before and during treatment with prochlorperazine.
Excipients.
This medicine contains lactose. If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicine.
Use during pregnancy or breastfeeding
Pregnancy.
There is no adequate evidence of the safety of prochlorperazine during human pregnancy. Prochlorperazine should be avoided during pregnancy unless considered essential by the physician. Since neuroleptics may prolong labor, the drug should be discontinued during labor until the cervix is 3-4 cm dilated. There is a risk of adverse effects of prochlorperazine on the newborn, which may be manifested by low Apgar scores, lethargy, or paradoxical excitement and tremor.
Since there is a risk of adverse reactions, including serious ones, including extrapyramidal symptoms and/or withdrawal symptoms (agitation, hypertension, hypotension, tremor, somnolence, respiratory distress or feeding disorders) in newborns whose mothers have taken antipsychotics, including prochlorperazine, during the third trimester of pregnancy, such children should be closely observed.
Breastfeeding period.
Since prochlorperazine may pass into breast milk, breastfeeding should be discontinued during treatment.
Ability to influence reaction speed when driving vehicles or other mechanisms
Patients should be warned about drowsiness in the first days of treatment. It is not recommended to drive a car or operate machinery.
Method of administration and doses
The drug is prescribed for adults. It is administered orally.
Prevention of nausea and vomiting
1-2 tablets (5-10 mg) 2-3 times a day.
Treatment of nausea and vomiting
4 tablets (20 mg) are taken immediately after the onset of symptoms, if necessary - another 2 tablets (10 mg) after 2 hours.
Dizziness
1 tablet (5 mg) 3 times a day. If necessary, the daily dose of the drug can be increased to 6 tablets (30 mg). After a few weeks, the daily dose can be gradually reduced to 1-2 tablets (5-10 mg).
As an adjunctive drug for the short-term treatment of anxiety disorders
1 tablet (5 mg) 3-4 times a day at the beginning of treatment. If necessary, the daily dose of the drug can be increased to 8 tablets (40 mg) in 3-4 doses.
Elderly patients
A lower daily dose of prochlorperazine is recommended for elderly patients.
Children
There is insufficient data on the use of prochlorperazine in children, therefore Vertinex® should not be prescribed to this age group of patients.
Overdose
Symptoms of overdose: drowsiness or loss of consciousness, hypotension, tachycardia, ECG changes, ventricular arrhythmia, hypothermia, extrapyramidal dyskinesia.
Treatment.
First aid. If no more than 6 hours have passed since the toxic dose of the drug was taken, gastric lavage should be performed. Inducing vomiting with the help of medications is not recommended. Activated charcoal should be given. There is no specific antidote. Supportive and symptomatic treatment is recommended.
Hypotension. In mild cases, elevation of the patient's upper extremities is sufficient. In severe cases, infusion therapy may be necessary to correct the total fluid volume. Pre-warmed infusion solutions should be administered (to prevent hypothermia). If fluid replacement is insufficient to correct hypotension, positive inotropic agents such as dopamine may be used.
The use of peripheral vasoconstrictors and adrenaline is not recommended.
CNS depression. Respiratory support medications should be used, including mechanical ventilation if necessary.
Dystonia. In severe cases, dystonia can be treated with procyclidine (5-10 mg) or orphenadrine (20-40 mg) intravenously or intramuscularly.
Convulsive syndrome. Diazepam should be administered intravenously.
Neuroleptic malignant syndrome. Physical cooling methods should be used. Dantrolene sodium may also be used.
Side effects
Immune system disorders: Type I hypersensitivity reactions, including angioedema and urticaria.
Blood and lymphatic system disorders: leukopenia, agranulocytosis.
Endocrine system: hyperprolactinemia, which can lead to galactorrhea; gynecomastia; amenorrhea; impotence; glucose intolerance; hyperglycemia.
Metabolic disorders: syndrome of inappropriate antidiuretic hormone secretion, hyponatremia.
Nervous system: acute dystonia or dyskinesia, including oculogyric crisis; akathisia; parkinsonian symptoms (tremor, rigidity, akinesia or others); tardive dyskinesia; insomnia; anxiety; convulsions.
From the organs of vision: ophthalmological changes.
Cardiac: ECG changes (QT interval prolongation, ST segment depression, U and/or T wave changes); cardiac rhythm disturbances (ventricular and atrial arrhythmias, atrioventricular block, ventricular tachycardia, which can lead to ventricular fibrillation or cardiac arrest); sudden death.
Vascular disorders: hypotension, venous thromboembolism (including cases of pulmonary embolism), deep vein thrombosis.
On the part of the digestive system: dry mouth.
Respiratory system: respiratory depression, nasal congestion.
Liver and biliary tract disorders: jaundice.
Skin and subcutaneous tissue disorders: metallic gray-purple discoloration of the skin; skin rashes; photosensitivity.
General disorders: neuroleptic malignant syndrome (hyperthermia, rigidity, autonomic dysfunction and impaired consciousness).
Expiration date
3 years.
Storage conditions
Store at a temperature not exceeding 25 °C.
Keep out of reach of children.
Packaging
10 tablets in a blister; 1 blister in a cardboard box.
10 tablets in a blister; 1 blister in a cardboard pack; 10 cardboard packs in a cardboard box.
Vacation category
According to the recipe.
Producer
KUSUM HEALTHCARE PVT LTD/KUSUM HEALTHCARE PVT LTD.
Address
SP-289 (A), RIICO Industrial area, Chopanki, Bhiwadi, Dist. Alwar (Rajasthan), India/SP-289 (A), RIICO Industrial area, Chopanki, Bhiwadi, Dist. Alwar (Rajasthan), India.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.