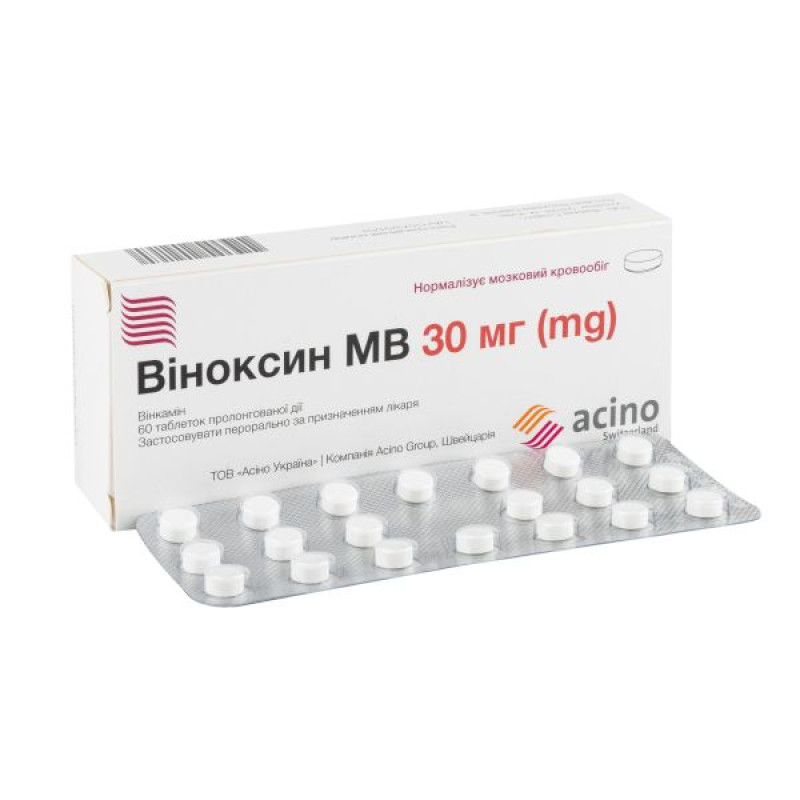
Vinoxin MV prolonged-release tablets 30 mg blister No. 60
Instructions Vinoksin MV prolonged-release tablets 30 mg blister No. 60
Composition
active ingredient: vincamine;
1 tablet contains vincamine 30 mg;
Excipients: lactose monohydrate; hypromellose; copovidone; colloidal silicon dioxide hydrophobic; colloidal silicon dioxide aqueous; magnesium stearate.
Dosage form
Extended-release tablets.
Main physicochemical properties: round, flat-cylindrical tablets with a bevel, white or almost white in color.
Pharmacotherapeutic group
Peripheral vasodilators. ATX code C04A X07.
Pharmacological properties
Pharmacodynamics
Vincamine has a selective vasoregulatory effect on cerebral blood flow, contributing to the adaptation of cerebral blood flow to the metabolic needs of the brain. The drug improves brain metabolism by enhancing glucose oxidation, thereby increasing energy production and contributing to an increase in the overall activity of the body. Vincamine increases the oxygen supply to neurons in a state of hypoxia. The drug reduces and stabilizes the peripheral resistance of cerebral vessels. It has been proven that vincamine does not have biological, hematological toxicity and side effects on the kidneys and liver.
Pharmacokinetics
It is rapidly absorbed from the digestive tract, the half-life is 60-90 minutes, and the binding to plasma proteins is 64%. Vincamine is completely metabolized in the liver, with only 4-6% excreted unchanged in the urine.
Indication
To normalize and adapt cerebral blood circulation according to the metabolic needs of the brain: to improve, regulate and maintain brain functions in the following conditions:
- memory impairment;
- impaired concentration;
- diabetic angiopathy;
- atherosclerotic damage to cerebral vessels;
- post-traumatic brain disorders;
- after acute cerebrovascular accident;
- cerebral disorders after cerebral ischemia;
- hypertensive encephalopathy;
- hearing and vision disorders of vascular origin;
- Disorientation in space and time, emotional disorders, which are consequences of various mental disorders.
Contraindication
Hypersensitivity to any component of the drug. Brain tumors (or diseases causing increased intracranial pressure), seizure disorders, acute stroke, cardiac arrhythmias, severe electrolyte disturbances (hypokalemia or hypocalcemia).
Interaction with other medicinal products and other types of interactions
Concomitant use with medicinal products that may induce torsades des pointes should be avoided. If this is not possible, close clinical and ECG monitoring is recommended.
Interactions specific to vinca alkaloid.
Quinupristin and dalfopristin are potent inhibitors of cytochrome P450 3A4 and may increase vinca alkaloid concentrations when used concomitantly. Patients should be monitored for vinca alkaloid adverse effects and the vincamine dose adjusted accordingly.
A possible mechanism of interaction between itraconazole and vinca alkaloid is inhibition of cytochrome P450 by itraconazole. Itraconazole may also inhibit the P-glycoprotein pump, which is responsible for the excretion of vinca alkaloid. Therefore, patients should be closely monitored when itraconazole and vincamine are used concomitantly.
Application features
Do not exceed recommended doses.
Use with caution in patients with myocardial infarction, heart disease or heart failure. In such cases, use low initial doses, conduct clinical supervision and ECG monitoring. Do not use in cases of prolongation of the QT interval, as there is a risk of developing ventricular arrhythmias.
With long-term use of vincamine, liver function should be monitored.
The drug contains lactose, so it should not be prescribed to patients with rare hereditary forms of galactose intolerance, lactase deficiency or glucose-galactose malabsorption syndrome.
Use during pregnancy or breastfeeding
Contraindicated.
Ability to influence reaction speed when driving vehicles or other mechanisms
Vincamine may affect your reaction time when driving or operating other machinery.
Method of administration and doses
Adults are prescribed 1 tablet orally 2 times a day. The duration of treatment is determined by the doctor individually depending on the course of the disease.
Children
Vinoksin MV tablets are not recommended for use in children.
Overdose
In case of overdose, close clinical observation, ECG monitoring, symptomatic treatment and support of vital functions are recommended. There is no specific antidote.
Adverse reactions
Mental disorders: slight increase in mental activity.
From the nervous system: headache, dizziness.
Cardiovascular system: QT interval prolongation, ventricular fibrillation (torsade des pointes).
Immune system disorders: hypersensitivity reactions, including angioedema.
Skin and subcutaneous tissue disorders: erythema, pruritus, allergic dermatitis, urticaria, papular rashes.
Expiration date
3 years.
Storage conditions
Store out of the reach of children, in the original packaging at a temperature not exceeding 25 °C.
Packaging
20 tablets in a blister; 1 or 3 blisters in a cardboard pack.
Vacation category
According to the recipe.
Producer
"Pharma Start" LLC.
Location of the manufacturer and its business address
Ukraine, 03124, Kyiv, I. Lepse Blvd., 8.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.