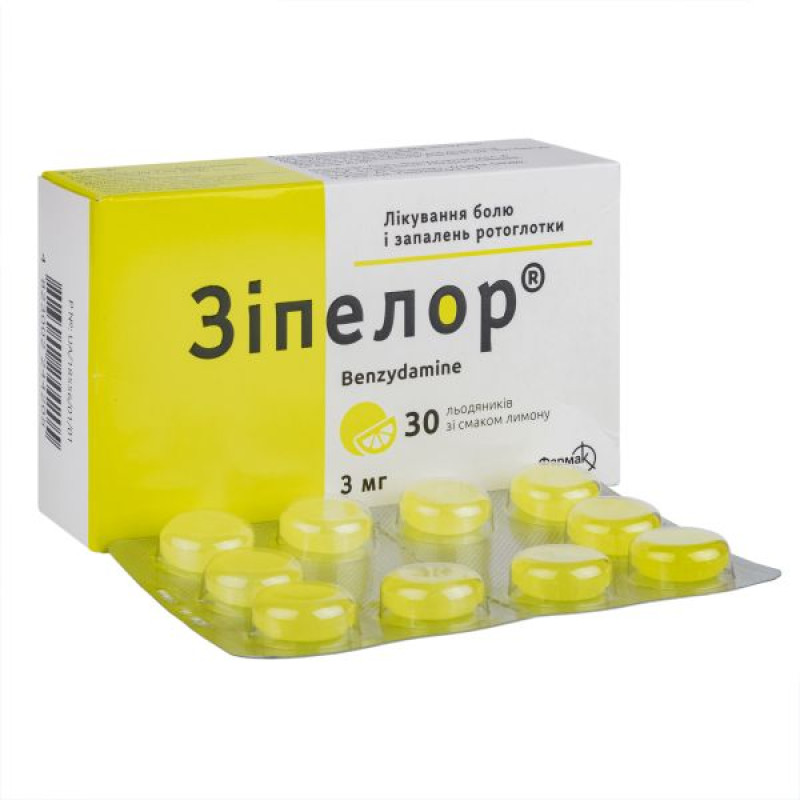
Zipelor lollipops 3mg lemon No. 30

Zipelor – lozenges for the symptomatic treatment of pain, irritation, and inflammation of the oropharynx.
Composition
active ingredient: benzydamine hydrochloride; 1 lollipop contains 3 mg of benzydamine hydrochloride, which is equivalent to 2.68 mg of benzydamine; excipients: isomalt (E 953); citric acid, monohydrate; aspartame (E 951); quinoline yellow (E 104); lemon flavoring; peppermint oil.Contraindication
Hypersensitivity to the active substance or to other components of the medicinal product.
Method of application
Lollipops should be slowly dissolved in the mouth.
Adults and children over 6 years of age: 1 lollipop 3 times a day.
Lozenges should not be swallowed. Lozenges should not be chewed.
The course of treatment should not exceed 7 days.
Application features
In some patients, oropharyngeal ulcers may be due to serious pathological processes. Therefore, patients whose symptoms worsen or do not improve within 3 days, or who develop a fever or other symptoms, should seek advice from a general practitioner or dentist as appropriate.
Benzydamine is not recommended for use in patients with hypersensitivity to acetylsalicylic acid or other nonsteroidal anti-inflammatory drugs.
The use of the drug may cause bronchospasm in patients with bronchial asthma or a history of bronchial asthma. The drug should be used with caution in such patients.
This medicinal product contains isomalt. If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicinal product.
This medicine contains aspartame, which is a derivative of phenylalanine, which is dangerous for patients with phenylketonuria.
Use during pregnancy or breastfeeding
Zipelor® should not be used during pregnancy or breastfeeding.
Children
This dosage form is used in children aged 6 years and older.
Children aged 6-11 years use this medicine under adult supervision.
Ability to influence reaction speed when driving vehicles or other mechanisms
When used in recommended doses, the drug has no effect on the ability to drive vehicles and operate machinery.
Overdose
There have been no reports of overdose with topical benzydamine.
However, it is known that benzydamine, when ingested in large doses (which exceeded the possible doses of this dosage form by hundreds of times), especially in children, caused agitation, convulsions, tremors, nausea, increased sweating, ataxia, vomiting. Such an acute overdose requires immediate gastric lavage, treatment of water and electrolyte balance disorders and symptomatic treatment, adequate hydration.
Adverse reactions
From the gastrointestinal tract: rarely - burning sensation in the mouth, dry mouth; frequency unknown - oral hypoesthesia.
On the part of the immune system: rarely - hypersensitivity reactions; frequency unknown - anaphylactic reactions.
Respiratory, thoracic and mediastinal disorders: very rarely - laryngospasm.
Skin and subcutaneous tissue disorders: uncommon - photosensitivity; very rare - angioedema.
Interaction with other drugs and other types of interactions.
No interaction studies have been conducted.
Storage conditions
Store in original packaging at a temperature not exceeding 25 ºС.
Keep out of reach of children.
Shelf life 3 years.
Do not use the drug after the expiration date indicated on the package.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.